
RELESTAT 0.5 mg/ml EYE DROPS SOLUTION

How to use RELESTAT 0.5 mg/ml EYE DROPS SOLUTION
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the Patient
Relestat 0.5 mg/ml Eye Drops Solution
epinastine hydrochloride
Read this entire leaflet carefully before starting to use this medication, as it contains important information for you.
- Keep this leaflet, as you may need to read it again. If you have any questions, ask your doctor or pharmacist.
- This medication has been prescribed to you only, and you should not give it to others, even if they have the same symptoms as you, as it may harm them.
- If you experience side effects, consult your doctor or pharmacist, even if they are not listed in this leaflet. See section 4.
Contents of the Package Leaflet
- What is Relestat and what is it used for
- What you need to know before using Relestat
- How to use Relestat
- Possible side effects
- Storage of Relestat
- Package Contents and Additional Information
1. What is Relestat and what is it used for
Relestat is an antiallergic medication.
Relestat is an eye drop solution used to treat the symptoms of seasonal allergic conjunctivitis, a seasonal allergic disease that affects the eye. The main symptoms treated by Relestat include tearing and itching, redness or swelling of the eyes or eyelids.
2. What you need to know before using Relestat
Do not use Relestat
- If you are allergic to epinastine hydrochloride or any of the other ingredients of this medication (listed in section 6).
Warnings and Precautions
Consult your doctor or pharmacist before starting to use Relestat:
- If you wear contact lenses, you should remove them before using Relestat. After use, wait at least 15 minutes before putting them back on. See also section 2, "Relestat contains benzalkonium chloride".
- If you need to use any other eye drops during treatment with Relestat: wait at least 10 minutes between application and the application of other eye drops.
Other Medications and Relestat
Tell your doctor or pharmacist if you are using, have recently used, or may need to use any other medication.
If you need to use any other eye drops during treatment with Relestat, follow the instructions included in "Warnings and Precautions" in this section.
Pregnancy and Breastfeeding
Pregnancy
If you are pregnant, think you may be pregnant, or plan to become pregnant, consult your doctor or pharmacist before using this medication.
Breastfeeding
It is not known if Relestat is excreted in breast milk. Consult your doctor or pharmacist before using this medication during the breastfeeding period.
Driving and Using Machines
You may experience temporary blurred vision after using Relestat. Do not drive or use machines until your vision is clear again.
Relestat contains benzalkonium chloride and phosphates
This medication contains 0.5 mg of benzalkonium chloride in each 5 ml of solution, equivalent to 0.1 mg/ml.
Benzalkonium chloride can be absorbed by soft contact lenses and may alter the color of the contact lenses. Remove the contact lenses before using this medication and wait 15 minutes before putting them back on.
Benzalkonium chloride may cause eye irritation, especially if you have dry eye or other corneal diseases (the transparent layer of the front of the eye). Consult your doctor if you feel a strange sensation, itching, or pain in the eye after using this medication.
This medication contains 23.75 mg of phosphate in each 5 ml of solution, equivalent to 4.75 mg/ml.
If you have severe corneal damage (the transparent layer of the front of the eye), treatment with phosphates, in very rare cases, may cause blurred vision due to calcium accumulation (see section 4).
3. How to use Relestat
Follow your doctor's instructions for administering this medication exactly. If you are unsure, consult your doctor or pharmacist again.
Use in Children
Relestat should not be used in children under 12 years of age.
Use in Adults and Adolescents (12 years or older)
The recommended dose is one drop in each eye that needs treatment, twice a day, for example, in the morning and at night.
You should use Relestat every day you have symptoms during the allergy season, but never for more than 8 weeks.
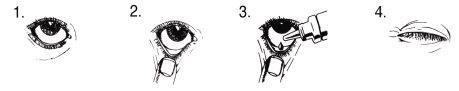
Instructions for Use
 You should not use this container if the security seal on the neck of the bottle is broken before its first use.
You should not use this container if the security seal on the neck of the bottle is broken before its first use.
Apply the drops as follows:
- Wash your hands. Tilt your head back and look at the ceiling.
- Gently separate the lower eyelid until a small gap appears.
- Turn the bottle upside down and squeeze it to let one drop fall into the eye that needs treatment.
- Release the lower eyelid and keep the eye closed for 30 seconds. If the drop falls outside the eye, try again.
To avoid contaminating the solution, avoid touching the dropper tip to the eye or any other surface.
Replace the cap and close the container immediately after use.
Wipe any excess liquid from your cheek with a clean tissue.
Correct application of the drops is very important. If you are unsure, consult your doctor or pharmacist.
If you use more Relestat than you should
If you use more drops of Relestat than you should, it is unlikely to cause you any harm. Apply the next drop(s) at the usual time. If you are concerned, talk to your doctor or pharmacist.
If you forget to use Relestat
If you forget a dose, apply it as soon as you remember, unless it is almost time for your next dose, in which case you should skip the missed dose. Then use your next dose at the usual time and continue with your regular schedule.
Do not use a double dose to make up for missed doses.
If you stop using Relestat
Relestat should be used as advised by your doctor.
If you have any further questions about using this medication, ask your doctor or pharmacist.
4. Possible Side Effects
Like all medications, this medication can cause side effects, although not everyone will experience them. It is likely that none of these effects will be serious; it is possible that most of them will be mild and only affect the eye.
If any of the following situations occur, stop using Relestat and contact your doctor immediately or go to the nearest hospital:
- asthma (an allergic disease that affects the lungs and causes breathing difficulties)
- if you experience symptoms of angioedema (swelling of the face, tongue, or throat; difficulty swallowing; hives; and difficulty breathing)
The following side effects may also occur:
Common(may affect up to 1 in 10 people)
- feeling of stinging or irritation of the eye (usually mild)
Uncommon(may affect up to 1 in 100 people)
General side effects:
- headache
- nasal congestion and irritation, which may cause runny nose or stuffy nose and sneezing
- unusual taste in the mouth
Side effects that affect the eye:
- redness of the eyes
- dryness of the eyes
- itching of the eyes
- difficulty seeing clearly
- eye discharge
Frequency not known(frequency cannot be estimated from available data):
- increased tear production
- eye pain
- allergic reaction affecting the eyes
- swelling of the eyes
- swelling of the eyelids
- skin rash and redness
Other side effects reported with phosphate-containing eye drops
This medication contains 23.75 mg of phosphate in each 5 ml of solution, equivalent to 4.75 mg/ml.
If you have severe corneal damage (the transparent layer of the front of the eye), treatment with phosphates, in very rare cases, may cause blurred vision due to calcium accumulation (see section 2).
Reporting Side Effects
If you experience any side effects, consult your doctor or pharmacist, even if they are not listed in this leaflet. You can also report them directly through the Spanish Medicines Agency's website: https://www.notificaRAM.es. By reporting side effects, you can help provide more information on the safety of this medication.
5. Storage of Relestat
Keep this medication out of the sight and reach of children.
Do not use this medication after the expiration date stated on the label and carton after EXP. The expiration date is the last day of the month indicated.
Do not use this medication if you notice that the eye drops show signs of deterioration, such as a change in color, and return the product to your pharmacist.
Do not store above 25°C.
Keep the bottle in its carton to protect it from light.
After opening the bottle, discard it 28 days later, even if there are still some drops left. This will protect you from infections. To help you remember, write the opening date on the carton.
Medications should not be disposed of via wastewater or household waste. Return the containers and medications you no longer need to the pharmacy's SIGRE collection point. Ask your pharmacist how to dispose of the containers and medications you no longer need. This will help protect the environment.
6. Package Contents and Additional Information
Composition of Relestat
- The active ingredient is epinastine hydrochloride. Each ml of eye drop solution contains 0.5 mg of epinastine hydrochloride.
- The other ingredients are benzalkonium chloride (preservative), disodium edetate, sodium chloride, sodium dihydrogen phosphate dihydrate, sodium hydroxide/hydrochloric acid (to adjust pH), and purified water.
Appearance of the Product and Package Contents
Relestat is a clear and colorless eye drop solution contained in a plastic bottle with a screw cap. Before opening, each bottle is filled to half its capacity and contains 5 ml of solution. Each package contains one bottle.
Marketing Authorization Holder and Manufacturer
Marketing Authorization Holder:
AbbVie Spain, S.L.U.
Avenida de Burgos 91,
28050 Madrid,
Spain
Manufacturer:
Allergan Pharmaceuticals Ireland
Castlebar Road
Westport
County Mayo
Ireland
This medication is authorized in the Member States of the European Economic Area under the following names:
Member State | Medicinal Product Name |
Austria, Belgium, Denmark, Germany, Greece, Hungary, Ireland, Italy, Luxembourg, Netherlands, Portugal, Spain, Sweden, United Kingdom | RELESTAT |
Czech Republic, France, Slovakia | PURIVIST |
Date of the last revision of this leaflet: April 2025
Detailed information about this medication is available on the website of the Spanish Agency for Medicines and Health Products (AEMPS) http://www.aemps.gob.es/
- Country of registration
- Average pharmacy price10.71 EUR
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to RELESTAT 0.5 mg/ml EYE DROPS SOLUTIONDosage form: EYEDROP, 0.5 mg/mlActive substance: azelastineManufacturer: Cooper Consumer Health B.V.Prescription requiredDosage form: EYEDROP, 1 mg/mlActive substance: olopatadineManufacturer: Tiedra Farmaceutica S.L.Prescription requiredDosage form: EYEDROP, 0.5 mg/mlActive substance: azelastineManufacturer: Qualix Pharma S.L.Prescription required
Online doctors for RELESTAT 0.5 mg/ml EYE DROPS SOLUTION
Discuss questions about RELESTAT 0.5 mg/ml EYE DROPS SOLUTION, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions















