REFIXIA 1000 IU POWDER AND SOLVENT FOR INJECTABLE SOLUTION

How to use REFIXIA 1000 IU POWDER AND SOLVENT FOR INJECTABLE SOLUTION
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the User
Refixia500UI powder and solvent for solution for injection
Refixia 1000UI powder and solvent for solution for injection
Refixia 2000UI powder and solvent for solution for injection
Refixia 3000UI powder and solvent for solution for injection
nonacog beta pegol
This medicine is subject to additional monitoring, which will allow for quick identification of new safety information. You can help by reporting any side effects you may get. The last section of this leaflet includes information on how to report side effects.
Read all of this leaflet carefully before you start using this medicine, because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the pack
- What is Refixia and what is it used for
- What you need to know before you use Refixia
- How to use Refixia
- Possible side effects
- Storage of Refixia
- Contents of the pack and other information
1. What is Refixia and what is it used for
What is Refixia
Refixia contains the active substance nonacog beta pegol. It is a long-acting factor IX. Factor IX is a protein that is naturally found in the blood and helps to stop bleeding.
What Refixia is used for
Refixia is used to treat and prevent bleeding episodes in patients of all ages with hemophilia B (congenital factor IX deficiency).
In patients with hemophilia B, factor IX is missing or does not work properly. Refixia replaces the missing or non-functioning factor IX and helps the blood to form clots at the site of bleeding.
2. What you need to know before you use Refixia
Do not use Refixia
- if you are allergic to the active substance or any of the other ingredients of this medicine (listed in section 6).
- if you are allergic to hamster proteins.
If you are in any of these situations or are not sure, consult your doctor before using this medicine.
Warnings and precautions
Traceability
It is important to keep a record of the batch number of Refixia. Therefore, each time you get a new pack of Refixia, you should write down the date and batch number (which appears on the packaging after Batch) and keep this information in a safe place.
Allergic reactions and development of inhibitors
There is a small risk that you may have a sudden and severe allergic reaction (e.g. anaphylactic reaction) to Refixia. Stop the injection and contact your doctor or emergency services immediately if you have signs of an allergic reaction, such as rash, hives, itching, swelling of the face, lips, tongue or throat, difficulty swallowing or breathing, shortness of breath, wheezing, chest tightness, pale and cold skin, palpitations and/or dizziness.
Your doctor may need to treat you quickly for these reactions. Your doctor may also do a blood test to check if you have developed inhibitors of factor IX (neutralizing antibodies) against your medicine, as inhibitors can develop together with allergic reactions. If you develop such inhibitors, you may have a higher risk of having severe allergic reactions (e.g. anaphylactic reactions) during future treatment with factor IX.
Due to the risk of having allergic reactions with factor IX, your initial treatment with Refixia should be carried out in a healthcare facility or in the presence of a healthcare professional who can provide you with the necessary medical care in case of an allergic reaction.
Tell your doctor immediately if bleeding does not stop as expected or if you need to significantly increase the amount of Refixia you need to stop a bleed. Your doctor will do a blood test to check if you have developed inhibitors (neutralizing antibodies) against Refixia. The risk of developing inhibitors is higher in people who have not been treated before with factor IX medicines, usually in young children.
Blood clots
Tell your doctor if you have any of the following, as the risk of blood clots increases during treatment with Refixia:
- you have recently had surgery
- you have any other serious illness, such as liver disease, heart disease or cancer
- you have risk factors for developing heart disease, such as high blood pressure, obesity or smoking.
Kidney disease (nephrotic syndrome)
There is a small risk of developing a specific kidney disease called "nephrotic syndrome" after administration of high doses of factor IX in patients with hemophilia B and factor IX inhibitors, as well as a history of allergic reactions.
Catheter-related complications
If you have a central venous access device (CVAD), you may develop infections or blood clots at the catheter insertion site.
Other medicines and Refixia
Tell your doctor if you are using, have recently used or might use any other medicines.
Pregnancy and breastfeeding
If you are pregnant or breastfeeding, think you may be pregnant or are planning to have a baby, ask your doctor for advice before using Refixia.
Driving and using machines
Refixia has no influence on the ability to drive and use machines.
Refixia contains sodium
This medicine contains less than 1 mmol of sodium (23 mg) per vial, which is essentially "sodium-free". In case of treatment with multiple vials, the total sodium content should be taken into account.
3. How to use Refixia
Treatment with Refixia should be started by a doctor who has experience in the treatment of patients with hemophilia B. Follow the instructions for administration of this medicine given by your doctor. If you are not sure, ask your doctor again how to use Refixia.
Your doctor will calculate your correct dose based on your weight and what the medicine is being used for.
Bleeding prevention
The usual dose of Refixia is 40 international units (IU) per kilogram of body weight. It is given by injection once a week. Your doctor may choose a different dose or change the frequency of injections based on your needs.
Bleeding treatment
The usual dose of Refixia is 40 international units (IU) per kilogram of body weight. Depending on the location and severity of the bleed, you may need a higher dose (80 IU per kilogram) or additional injections. Consult your doctor for the dose and number of injections you need.
Use in children and adolescents
Refixia can be used in children and adolescents of all ages. The dose in children and adolescents is also calculated based on body weight and is the same dose as for adults.
How Refixia is administered
Refixia is available as a powder and solvent for solution for injection (reconstitution) and should be injected into a vein. See "Instructions for using Refixia" for more information.
If you use more Refixia than you should
If you use more Refixia than you should, contact your doctor.
If you need to significantly increase the amount of Refixia you need to stop a bleed, tell your doctor immediately. For more information, see section 2 "Allergic reactions and development of inhibitors".
If you forget to use Refixia
If you forget a dose, inject the missed dose as soon as you remember. Do not inject a double dose to make up for the missed dose. If you are not sure, ask your doctor.
If you stop using Refixia
If you stop using Refixia, you will no longer be protected against bleeding or an existing bleed may not stop. Do not stop using Refixia without talking to your doctor first.
If you have any other questions about the use of this medicine, ask your doctor.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Allergic reactions may occur with this medicine.
If you have a sudden and severe allergic reaction (e.g. anaphylactic reaction), stop the injection immediately. If you have any of the following early signs of a severe allergic reaction (anaphylactic reaction), contact your doctor or emergency services immediately:
- difficulty swallowing or breathing
- shortness of breath or wheezing
- chest tightness
- swelling of the face, lips, tongue or throat
- rash, hives, itching or prickling sensation
- pale and cold skin, palpitations and/or dizziness (low blood pressure).
For children not previously treated with factor IX medicines, the development of inhibitors (see section 2) is common (up to 1 in 10 patients). If this happens, the medicine may stop working properly and your child may experience persistent bleeding. If this happens, you should contact your doctor immediately.
The following side effects have been observed with Refixia:
Common side effects(may affect up to 1 in 10 people)
- allergic reactions (hypersensitivity). This can be severe and life-threatening (anaphylactic reactions)
- itching (pruritus)
- skin reactions at the injection site
- nausea
- feeling very tired
- rash
- children not previously treated with factor IX medicines: neutralizing antibodies (inhibitors), anaphylactic reactions.
Uncommon side effects(may affect up to 1 in 100 people)
- palpitations
- flushing.
Reporting of side effects
If you get any side effects, talk to your doctor, pharmacist or nurse. This includes any possible side effects not listed in this leaflet. You can also report side effects directly via the national reporting system listed in Appendix V. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of Refixia
Keep this medicine out of the sight and reach of children.
Do not use Refixia after the expiry date which is stated on the carton and on the label of the vial and the pre-filled syringe after "EXP". The expiry date refers to the last day of the month stated.
Store in a refrigerator (2°C to 8°C). Do not freeze. Keep the vial in the outer packaging to protect it from light.
Refixia can be stored at room temperature (up to 30°C) for a maximum of 1 year. Write the date you removed Refixia from the refrigerator and stored it at room temperature on the carton. The new expiry date should never be later than the one stated on the carton. Discard this medicine if you have not used it before the new expiry date. After storing the medicine at room temperature, do not put it back in the refrigerator.
Use the injection immediately after preparing the solution (reconstitution). If you cannot use it immediately, you must use it within 24 hours if stored in a refrigerator (2°C to 8°C) or within 4 hours if stored at room temperature (up to 30°C).
The powder in the vial is a white to off-white powder. If the color of the powder has changed, do not use it.
The reconstituted solution is clear and colorless to slightly yellow. Do not use the reconstituted solution if you notice it contains particles or discoloration.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. This will help protect the environment.
6. Container Contents and Additional Information
Composition of Refixia
- The active substance is nonacog beta pegol (human coagulation factor IX [rDNA] pegylated). Each vial of Refixia contains 500 IU, 1,000 IU, 2,000 IU, or 3,000 IU of nonacog beta pegol, which corresponds to approximately 125 IU/ml, 250 IU/ml, 500 IU/ml, or 750 IU/ml, respectively, after reconstitution with the histidine solvent.
- The other components of the powder are sodium chloride, histidine, sucrose, polysorbate 80, mannitol, sodium hydroxide, and hydrochloric acid. See section 2 "Refixia contains sodium".
- The components of the sterilized solvent are histidine, water for injectable preparations, sodium hydroxide, and hydrochloric acid.
Appearance of Refixia and Container Contents
- Refixia is supplied as a powder and solvent for solution for injection (500 IU, 1,000 IU, 2,000 IU, or 3,000 IU of powder in a vial and 4 ml of solvent in a pre-filled syringe, a plunger rod with a vial adapter; package size of 1).
- The powder is white to off-white and the solvent is clear and colorless.
Marketing Authorization Holder and Manufacturer
Novo Nordisk A/S
Novo Allé
DK-2880 Bagsværd, Denmark
Date of Last Revision of this Leaflet:
Other Sources of Information
Detailed information on this medicinal product is available on the European Medicines Agency website: http://www.ema.europa.eu.
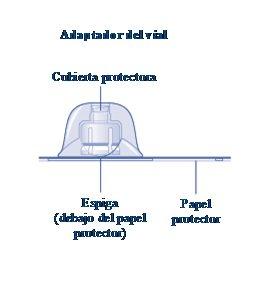
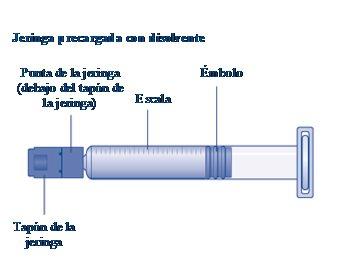
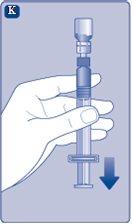
Instructions for UseRefixia Read these instructions carefully before using Refixia. Refixia is supplied as a powder. Before injection, a solution (reconstituted) must be prepared with the supplied solvent in the syringe. The solvent is a histidine solution. The reconstituted solution must be injected into a vein (intravenous [IV] injection). The components of this package are designed to reconstitute and inject Refixia. You will also need an intravenous infusion set (tubing and butterfly needle), sterile alcohol swabs, gauze, and band-aids. These materials are not included in the Refixia package. Do not use the equipment without proper training from your doctor or nurse. Always wash your hands and ensure the area around you is clean. When preparing and injecting the medication directly into the veins, it is essential to use a clean and germ-free (aseptic) technique.Incorrect technique can introduce germs that can infect the blood. Do not open the equipment until you are ready to use it. Do not use the equipment if it has been dropped or is damaged.Use a new package instead. Do not use the equipment if it has expired.Use a new package instead. The expiration date is printed on the outer packaging, the vial, the vial adapter, and the pre-filled syringe. Do not use the equipment if you suspect it is contaminated.Use a new package instead. Do not discard any components until the reconstituted solution has been injected. The equipment is for single use. | |
Contents The package contains:
| |
| |
|
|
|
|
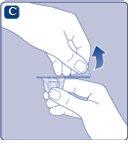
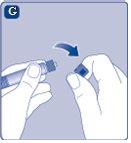
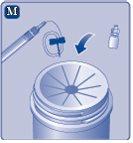
If the protective paper is not fully sealed or is broken, do not use the vial adapter. Do not remove the vial adapter from the protective cover with your fingers.If you touch the spike of the vial adapter, you can transfer germs from your fingers. |
|
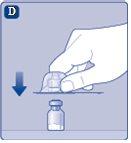
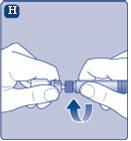
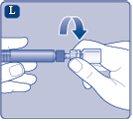
Once attached, do not remove the vial adapter from the vial. |
|
Remove the protective coverfrom the vial adapter. Do not remove the vial adapter from the vialwhen removing the protective cover. |
|
|
|
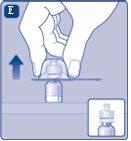
If the syringe cap is loose or missing, do not use the pre-filled syringe. |
|
|
|
|
|
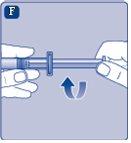
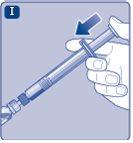
Do not shake the vial, as this would produce foam.
|
|
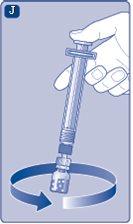
It is recommended to use Refixia immediately after reconstitution,as, if left, the medication may become non-sterile and could cause infections. If you cannot use the reconstituted Refixia solution immediately,you must use it within 4 hours if stored at room temperature (up to 30°C) and within 24 hours if stored in the refrigerator (between 2°C and 8°C). Store the reconstituted product in the vial. Do not freeze the reconstituted Refixia solution or store it in syringes. Store the reconstituted Refixia solution away from direct light. If your dose requires more than one vial, repeat steps Ato Jwith additional vials, vial adapters, and pre-filled syringes until the required dose is reached. | |
If there is air in the syringe at any point, inject the air back into the vial.
|
|
|
|
Refixia is now ready to be injected into a vein.
Injection of Refixia through needleless connectors for intravenous (IV) catheters Precaution:the pre-filled syringe is made of glass and is designed to be compatible with standard luer-lock connections. Some needleless connectors with an internal spike are incompatible with the pre-filled syringe. This incompatibility can prevent medication administration and/or damage the needleless connector. Injection of the solution through a central venous access device (CVAD) such as a central venous catheter or subcutaneous port:
| |
Disposal
Do not throw it away in household trash. |
|
Do not disassemble the equipment before disposal. Do not reuse the equipment. |
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to REFIXIA 1000 IU POWDER AND SOLVENT FOR INJECTABLE SOLUTIONDosage form: INJECTABLE, 1,000 IUActive substance: coagulation factor IXManufacturer: Swedish Orphan Biovitrum Ab (Publ)Prescription requiredDosage form: INJECTABLE, 2,000 IUActive substance: coagulation factor IXManufacturer: Swedish Orphan Biovitrum Ab (Publ)Prescription requiredDosage form: INJECTABLE, 250 IUActive substance: coagulation factor IXManufacturer: Swedish Orphan Biovitrum Ab (Publ)Prescription required
Online doctors for REFIXIA 1000 IU POWDER AND SOLVENT FOR INJECTABLE SOLUTION
Discuss questions about REFIXIA 1000 IU POWDER AND SOLVENT FOR INJECTABLE SOLUTION, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions