
Villfact 500 i.m.
Ask a doctor about a prescription for Villfact 500 i.m.

How to use Villfact 500 i.m.
LEAFLET INCLUDED IN THE PACKAGE: INFORMATION FOR THE USER
Willfact 500 IU
powder and solvent for solution for injection
Willfact 1000 IU powder and solvent for solution for injection
Willfact 2000 IU powder and solvent for solution for injection
human von Willebrand factor
You should carefully read the contents of this leaflet before using the medicine because it contains
important information for the patient.
- You should keep this leaflet, so you can read it again if you need to.
- If you have any further questions, you should ask your doctor, pharmacist, or nurse.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their symptoms are the same as yours.
- If you experience any side effects, including those not listed in this leaflet, you should tell your doctor, pharmacist, or nurse. See section 4.
Table of contents of the leaflet
- 1. What is Willfact and what is it used for
- 2. Important information before using Willfact
- 3. How to use Willfact
- 4. Possible side effects
- 5. How to store Willfact
- 6. Contents of the pack and other information
1. What is Willfact and what is it used for
Willfact is made from human plasma (the liquid part of the blood) and contains the active substance human von Willebrand factor (vWF).
vWF is involved in blood clotting. Lack of this factor, as in von Willebrand disease, means that blood does not clot as quickly as it should, resulting in an increased tendency to bleed. Replacing vWF with Willfact temporarily corrects the blood clotting mechanism.
Willfact is indicated for the prevention of bleeding associated with surgical procedures and other bleeding, as well as treatment in patients with von Willebrand disease, if treatment with desmopressin (DDAVP) is ineffective or contraindicated.
Willfact can be used in all age groups.
Willfact should not be used to treat hemophilia A.
2. Important information before using Willfact
When not to use Willfact
- If you are allergic to human von Willebrand factor or any of the other ingredients of this medicine (listed in section 6).
- If you have been diagnosed with hemophilia A.
Warnings and precautions
Treatment with Willfact should always be supervised by a doctorexperienced in the treatment of bleeding disorders.
If you have severe bleeding and blood tests confirm a decrease in factor VIII, you will receive a factor VIII concentrate and an additional vWF preparation within the first 12 hours.
Allergic reactions
There may be hypersensitivity reactions, including allergic reactions, similar to those that occur with other protein-based intravenous drugs derived from human blood or plasma.
You will be monitored during injection to detect early signs of hypersensitivity.
These include rash (hives or generalized urticaria), feeling of chest tightness, wheezing, decreased blood pressure (hypotension), and severe allergic reactions (anaphylaxis).
Your doctor will inform you about the warning signs of an allergic reaction.
If you experience any objective or subjective signs of hypersensitivity, treatment should be discontinued and medical help sought immediately.
Viral safety
Certain measures are taken to prevent the transmission of infections to patients when producing medicines from human blood or plasma. These include:
- careful selection of blood and plasma donors, allowing the exclusion of individuals at risk of infection,
- testing of each donated batch and plasma pool for the presence of viruses/infections,
- introduction of steps in the processing of blood or plasma that allow the inactivation or removal of viruses.
Despite these measures, it cannot be completely ruled out that the transmission of infection may occur when administering drugs produced from human blood or plasma. This also applies to unknown or newly emerging viruses or other types of infections.
The measures taken are considered effective against enveloped viruses, such as human immunodeficiency virus (HIV, which causes AIDS), hepatitis B virus, and hepatitis C virus.
The measures taken have limited value against non-enveloped viruses, such as hepatitis A virus and parvovirus B19. Infection with parvovirus B19 can be serious in pregnant women (as there is a risk of infection of the unborn child) and patients with weakened immune systems or certain types of anemia (e.g., sickle cell anemia or hemolytic anemia).
Vaccinations
Your doctor may recommend that you receive vaccinations against hepatitis A and hepatitis B if you regularly/repeatedly receive human von Willebrand factor produced from plasma.
Recording the batch number
It is strongly recommended that when administering each dose of Willfact, the patient's name and batch number of the medicine be recorded to document which batches of the medicine were administered.
Risk of thrombosis
There may be a blockage of blood vessels by blood clots (thrombosis). The risk is particularly high if certain risk factors have been identified in the medical history or laboratory tests. In such cases, the patient's condition will be closely monitored for early signs of thrombosis. Measures to prevent the blockage of blood vessels by blood clots should also be taken.
The doctor using factor VIII containing von Willebrand factor should be aware that continued treatment may cause excessive increase in factor VIII activity. If the patient receives a vWF preparation containing factor VIII, the doctor should regularly monitor factor VIII activity in the blood. This will protect the patient from excessive factor VIII activity in the blood, which may increase the risk of thrombotic events.
Limitation of efficacy
In patients with von Willebrand disease (especially type 3), proteins that neutralize the action of vWF may be formed. These proteins are called neutralizing antibodies or inhibitors. The doctor will check if inhibitors of vWF are formed in the patient's body if laboratory test results show a lack of proper restoration of vWF concentrations or if bleeding does not decrease despite the use of the appropriate dose of Willfact. If there is a high level of inhibitors, vWF treatment may not be effective. In such cases, other treatment methods should be considered. New therapy will be performed by a doctor experienced in the treatment of bleeding disorders.
Willfact and other medicines
Tell your doctor or pharmacist about all medicines you are taking or have recently taken, as well as any medicines you plan to take.
Pregnancy and breastfeeding
Willfact can be used in pregnant or breastfeeding women only if it is clearly necessary.
No controlled clinical trials have been conducted to assess the safety of using Willfact in pregnant or breastfeeding women, and animal studies are not sufficient to confirm safety in the context of fertility, pregnancy, and child development during pregnancy and after birth.
If you are pregnant or breastfeeding, think you may be pregnant, or plan to have a child, you should consult your doctor or pharmacist before using this medicine.
Driving and using machines
No effect on the ability to drive or use machines has been observed.
Willfact contains sodium
One vial of 5 ml (500 IU) of Willfact contains 0.15 mmol (3.4 mg) of sodium.
This corresponds to 0.17% of the recommended maximum daily intake of sodium for an adult.
One vial of 10 ml (1000 IU) of Willfact contains 0.3 mmol (6.9 mg) of sodium.
This corresponds to 0.35% of the recommended maximum daily intake of sodium for an adult.
One vial of 20 ml (2000 IU) of Willfact contains 0.6 mmol (13.8 mg) of sodium.
This corresponds to 0.69% of the recommended maximum daily intake of sodium for an adult.
3. How to use Willfact
Treatment should be initiated and monitored by a doctor experienced in the treatment of bleeding disorders.
If the doctor considers that you can administer the medicine at home, they will instruct you accordingly.
Dosage
This medicine should always be used exactly as your doctor has told you. If you are not sure, ask your doctor.
It is recommended that Willfact be administered by a doctor or nurse. However, if you have been given Willfact to use at home, your doctor will ensure that you are informed about how to perform the injection and the correct dose. You should follow the doctor's instructions and ask for help if you have problems using the syringe. The syringe can only be used by a trained person.
Your doctor will calculate the correct dose of Willfact (expressed in international units - IU).
The dose depends on:
- body weight,
- site of bleeding,
- severity of bleeding,
- patient's clinical condition,
- necessity of surgery,
- von Willebrand factor activity in the blood after surgery,
- severity of the disease.
The dose is in the range of 40–80 IU/kg body weight.
Your doctor will recommend that you have blood tests to check:
- factor VIII (FVIII:C) levels,
- von Willebrand factor (vWF:RCo) levels,
- presence of inhibitors,
- early signs of clot formation - in patients at risk of such complications.
Based on the results of these tests, your doctor may adjust the frequency of injections and the dose administered.
In some cases, it may be necessary to use a factor VIII preparation (another coagulation protein) in addition to Willfact to allow for faster treatment or to prevent bleeding (in emergency situations or in the case of acute bleeding).
Willfact can also be used for long-term prophylaxis, in which case the dose is also determined individually. Administration of Willfact at a dose of 40–60 IU/kg body weight 2-3 times a week reduces the number of bleeding episodes.
If you think that Willfact is too strong or too weak, you should consult your doctor.
Use in children and adolescents
Dosing in children and adolescents is based on body weight. In some cases, especially in younger patients (under 6 years), higher doses (up to 100 IU/kg body weight) may be necessary.
Method of administration
Detailed instructions for reconstitution and administration of the medicinal product are given at the end of the leaflet.
Overdose
No symptoms of overdose with Willfact have been reported; however, in the event of administration of a large amount, the risk of thrombosis cannot be ruled out.
Missed dose
If you miss a dose of Willfact, you should consult your doctor.
Do not take a double dose to make up for a missed dose.
If you have any further questions about using this medicine, you should ask your doctor, pharmacist, or nurse.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
You should contact your doctor immediately in the following cases:
- Occurrence of hypersensitivity symptoms or allergic reactions (observed not very often, may affect up to 1 in 100 patients). In some cases, such reactions may develop into a severe allergic reaction (anaphylaxis), including anaphylactic shock (observed with unknown frequency).
Warning signs of an allergic reaction include:
- difficulty breathing and swallowing,
- wheezing,
- chest tightness,
- rapid heartbeat,
- decreased blood pressure,
- fainting,
- extreme fatigue,
- anxiety, nervousness,
- headache,
- chills, feeling of cold,
- flushes,
- swelling of various parts of the body,
- skin rash, generalized urticaria,
- feeling of burning and stinging at the injection site,
- tingling,
- nausea and vomiting.
If you experience any of these symptoms, you should stop treatment immediately and inform your doctorto initiate appropriate treatment, depending on the type and severity of the reaction.
- Decreased efficacy of the medicine (lack of bleeding control). This may be due to inhibition of von Willebrand factor (observed with unknown frequency).
In patients with von Willebrand disease, especially type 3, proteins that neutralize the action of vWF may be formed. These proteins are called neutralizing antibodies or inhibitors. Patients treated with vWF should be closely monitored by the doctor for the development of inhibitors through appropriate clinical observation and laboratory tests. If inhibitors develop, this condition may manifest as an inadequate clinical response. It may also co-occur with severe allergic reactions.
- Occurrence of signs of circulatory disorders in the limbs (e.g., cold and pale limbs) or vital organs (e.g., severe chest pain). This may be due to the formation of blood clots in blood vessels (observed with unknown frequency).
There is a risk of blood clot formation (thrombosis), especially in patients at risk. After correcting the von Willebrand factor deficiency, patients should be monitored for early signs of thrombosis or disseminated intravascular coagulation and receive prophylactic treatment to prevent thrombosis in situations where the risk of its occurrence is increased (after surgery, in bedridden patients, in patients with a deficiency of a coagulation inhibitor or fibrinolytic enzyme).
If you are taking vWF preparations containing FVIII, the risk of thrombosis may be higher due to persistently elevated FVIII levels in the blood.
The following side effects are common(may affect up to 1 in 10 patients):
- reactions at the injection site.
The following side effects are uncommon(may affect up to 1 in 100 patients):
- dizziness,
- paresthesia, numbness,
- flushes,
- itching,
- feeling of chest tightness,
- chills, feeling of cold.
The following side effects have been reported with an unknown frequency:
- fever.
Reporting side effects
If you experience any side effects, including those not listed in this leaflet, you should tell your doctor, pharmacist, or nurse. Side effects can be reported directly to the Department of Drug Safety Monitoring of the Office for Registration of Medicinal Products, Medical Devices, and Biocidal Products:
Jerozolimskie Avenue 181C
02-222 Warsaw
Phone: +48 22 49 21 301
Fax: +48 22 49 21 309
Website: https://smz.ezdrowie.gov.pl
Side effects can also be reported to the marketing authorization holder.
Reporting side effects will help to gather more information on the safety of this medicine.
5. How to store Willfact
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date stated on the vial label and carton.
Do not store above 25°C. Store in the original package to protect from light.
Do not freeze.
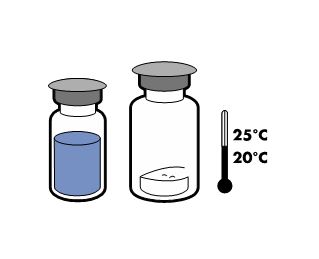
To maintain sterility, the product should be used immediately after reconstitution. However, the chemical and physical stability of the product has been demonstrated after storage for 24 hours at 25°C.
Do not use this medicine if you notice turbidity or solid particles in the solution.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. This will help protect the environment.
6. Contents of the pack and other information
What Willfact contains
The active substanceis human von Willebrand factor (500 IU, 1000 IU, 2000 IU) expressed in international units of ristocetin cofactor activity (vWF:RCo).
After reconstitution with 5 ml (500 IU), 10 ml (1000 IU), or 20 ml (2000 IU) of water for injection, one vial contains approximately 100 IU/ml of human von Willebrand factor.
Before the addition of albumin, the specific activity of the solution is at least 60 IU vWF:RCo per 1 mg of total protein.
Other ingredients:
Powder: human albumin, arginine hydrochloride, glycine, sodium citrate, and calcium chloride dihydrate.
Solvent: water for injection.
What Willfact looks like and contents of the pack
Willfact is provided as a white or pale yellow powder or a brittle solid and a clear, colorless solvent for solution for injection after reconstitution using a transfer system.
The available pack sizes of Willfact are 500 IU/5 ml, 1000 IU/10 ml, 2000 IU/20 ml.
The reconstituted solution should be clear or slightly opalescent, colorless or slightly yellow.
Marketing authorization holder and manufacturer
LFB-BIOMEDICAMENTS
3, avenue des Tropiques,
ZA de Courtaboeuf,
91940 LES Ulis,
FRANCE
[email protected]
Manufacturers
LFB-BIOMEDICAMENTS
3, avenue des Tropiques,
ZA de Courtaboeuf,
91940 LES Ulis,
FRANCE
LFB-BIOMEDICAMENTS
59 Rue de Trévise
59000 LILLE
FRANCE
This medicine is authorized in the Member States of the European Economic Area and in the United Kingdom (Northern Ireland) under the following names:
Austria
Willfact
Czech Republic
WILLFACT
Denmark
Willfact
Spain
Willfact
Germany
WILLFACT
Norway
Willfact
Poland
Willfact
Slovakia
Willfact
Sweden
Willfact
Hungary
Willfact
United Kingdom (Northern Ireland)
Willfact
Date of last revision of the leaflet:
- 01.2024 ________________________________________________________________________________________
INSTRUCTIONS FOR USE:
Dosage
Generally, the administration of 1 IU/kg body weight of von Willebrand factor results in an increase in circulating vWF:RCo activity of approximately 0.02 IU/ml (2%).
The aim is to achieve vWF:RCo levels of >0.6 IU/ml (60%) and FVIII:C levels of >0.4 IU/ml (40%).
Hemostasis is uncertain until factor VIII coagulant activity (FVIII:C) reaches 0.4 IU/ml (40%). Administration of von Willebrand factor alone does not result in immediate maximum increase in FVIII:C activity for at least 6–12 hours. Therefore, if the initial FVIII:C activity is below the critical value and rapid correction of hemostasis is necessary, such as in the case of bleeding, severe trauma, or emergency surgery, factor VIII should be administered with the first dose of von Willebrand factor to achieve hemostatic levels of FVIII:C in the blood.
- Initiation of treatment: The first dose of Willfact is 40-80 IU/kg body weight for the treatment of bleeding or trauma. It is administered in combination with the required amount of factor VIII preparation, calculated based on the patient's initial FVIII:C activity in the blood, to achieve the desired FVIII:C activity in the blood, immediately before surgery or as soon as possible after the start of a bleeding episode or severe trauma. In the case of scheduled surgery, the first injection of Willfact should be administered 1 hour before surgery. Administration of the initial dose of 80 IU/kg body weight of Willfact may be necessary, especially in patients with type 3 von Willebrand disease, where maintaining adequate activity may require higher doses than in other types of vWD.
In the case of scheduled surgery, the first injection of Willfact should be administered 12–24 hours before surgery, and the second injection should be administered immediately before surgery. In such cases, concurrent administration of factor VIII preparation is not necessary, as the endogenous FVIII:C level usually reaches the critical value of 0.4 IU/ml (40%) before surgery. However, this should be confirmed in each patient.
- Subsequent injections: If necessary, treatment with Willfact should be continued as monotherapy at a dose of 40-80 IU/kg body weight per day in 1 or 2 injections per day for one to several days. The dose and frequency of injections should be adjusted according to the type of surgery, the patient's clinical and biological condition (vWF:RCo and FVIII:C), and the type and severity of the bleeding episode.
- Long-term prophylaxis: Willfact can be administered for long-term prophylaxis at a dose adjusted individually for each patient. Administration of Willfact at a dose of 40-60 IU/kg body weight 2-3 times a week reduces the number of bleeding episodes.
- Home treatment: With the doctor's consent, especially in the case of minor or moderate bleeding or long-term prophylaxis of bleeding, home treatment can be initiated.
Children and adolescents
The dose for each indication is based on body weight. The dose and duration of treatment should be adjusted according to the patient's clinical condition and vWF:RCo and FVIII:C levels in the blood.
- Initiation of treatment
- In children under 6 years of age, the initial dose may be determined based on the patient's incremental recovery (IR) or, if IR data are not available, the initial dose may be 60-100 IU/kg body weight to increase the patient's vWF:RCo level to 100 IU/dl.
- In children over 6 years of age and adolescents, the dosing is the same as for adult patients.
- Subsequent injections: In children and adolescents, subsequent doses should be determined individually based on the clinical condition and vWF:RCo level and adjusted according to the clinical response.
Scheduled surgery
- In children under 6 years of age, a second dose can be administered 30 minutes before surgery after the first dose given 12-24 hours before surgery.
- In children over 6 years of age and adolescents, the dosing is the same as for adult patients.
- Prophylaxis: In children and adolescents, the dose and frequency of re-administration should be determined individually based on the incremental recovery and vWF:RCo level in the patient and adjusted according to the clinical response.
Method and route of administration
Intravenous administration
Reconstitution
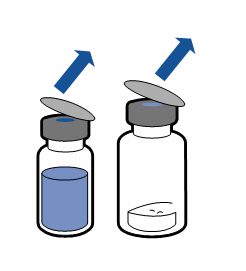
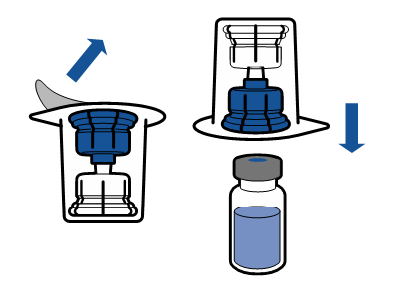
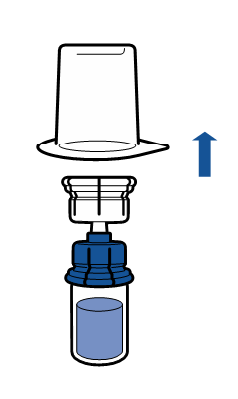
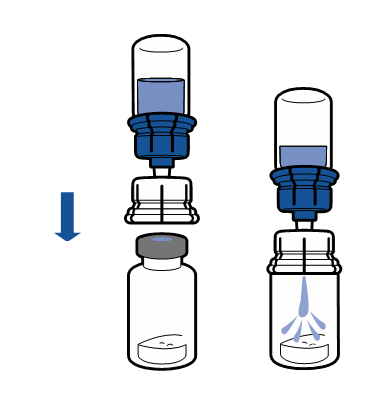
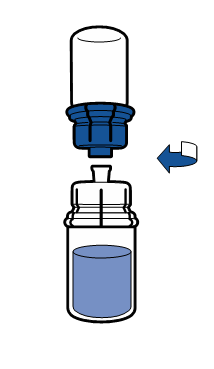
Current aseptic technique recommendations should be followed. The transfer system is only used for reconstitution of the medicine, as described below. The system is not intended for administration of the medicine to the patient.
  |
|
    |
|
The powder should dissolve within 5 minutes, usually instantly.
The resulting solution should be clear or slightly opalescent, colorless or slightly yellow. Before administration, the reconstituted product should be visually inspected for particulate matter and discoloration.
Do not use a cloudy or precipitated solution.
Do not mix with other medicines.
Do not dilute the reconstituted product.
Administration
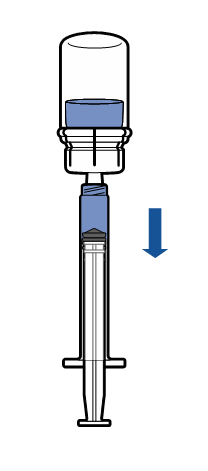 |
|
Storage after reconstitution
To maintain sterility, the product should be used immediately after reconstitution. However, the chemical and physical stability of the product has been demonstrated after storage for 24 hours at 25°C.
Any unused product or waste material should be disposed of in accordance with local requirements.
- Country of registration
- Active substance
- Prescription requiredNo
- Manufacturer
- ImporterLFB-Biomedicamensts LFB-Biomedicaments
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to Villfact 500 i.m.Dosage form: Powder, 1000 IUActive substance: von Willebrand factorManufacturer: LFB-Biomedicamensts LFB-BiomedicamentsPrescription not requiredDosage form: Powder, 2000 IU/20 mlActive substance: von Willebrand factorManufacturer: LFB-Biomedicamensts LFB-BiomedicamentsPrescription not requiredDosage form: Powder, 1000 IUActive substance: coagulation factor VIIIManufacturer: CSL Behring GmbHPrescription required
Alternatives to Villfact 500 i.m. in other countries
The best alternatives with the same active ingredient and therapeutic effect.
Alternative to Villfact 500 i.m. in Spain
Online doctors for Villfact 500 i.m.
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for Villfact 500 i.m. – subject to medical assessment and local rules.














