
Trusopt

Ask a doctor about a prescription for Trusopt

How to use Trusopt
Package Leaflet: Information for the User
TRUSOPT, 20 mg/ml, Eye Drops, Solution
Dorzolamide
Read the Package Leaflet Carefully Before Using the Medication, as it Contains Important Information for the Patient
- Keep this package leaflet, you may need to read it again.
- In case of any doubts, consult a doctor or pharmacist.
- This medication has been prescribed for a specific person. Do not pass it on to others. The medication may harm another person, even if their symptoms are the same.
- If the patient experiences any side effects, including any side effects not listed in this leaflet, they should inform their doctor or pharmacist. See section 4.
Table of Contents of the Package Leaflet
- 1. What is Trusopt and what is it used for
- 2. Important information before using Trusopt
- 3. How to use Trusopt
- 4. Possible side effects
- 5. How to store Trusopt
- 6. Contents of the package and other information
1. What is Trusopt and what is it used for
Trusopt is a medication containing dorzolamide hydrochloride, which belongs to carbonic anhydrase inhibitors. Trusopt is used locally in the conjunctival sac. Trusopt is recommended to reduce increased intraocular pressure and to treat glaucoma.
2. Important information before using Trusopt
When Not to Use Trusopt
- if the patient is allergic to the active substance or any of the other ingredients of this medication (listed in section 6).
Warnings and Precautions
Before starting to use Trusopt, discuss it with a doctor or pharmacist. Inform the doctor about past and current diseases and allergies to medications. In case of eye irritation or new symptoms, such as eye redness or eyelid swelling, seek medical attention immediately. If the patient suspects that an allergic reaction has occurred after using Trusopt (e.g., rash, severe skin reaction, or itching), they should stop using the medication and seek medical attention immediately. When using contact lenses, remove them before administering the medication and wait at least 15 minutes before putting them back on.
Trusopt and Other Medications
Inform the doctor or pharmacist about all medications currently being used or recently used, as well as medications planned to be taken, including other eye drops and over-the-counter medications. It is essential to inform the doctor about taking high doses of acetylsalicylic acid or sulfonamides.
Children and Adolescents
Studies have been conducted on the use of Trusopt in infants and children under 6 years of age with increased intraocular pressure or glaucoma. For additional information, consult a doctor.
Elderly Patients
No significant differences in efficacy and safety of Trusopt have been observed in elderly patients compared to younger patients.
Pregnancy and Breastfeeding
If the patient is pregnant or breastfeeding, thinks they may be pregnant, or plans to have a child, they should consult a doctor before using this medication. The doctor will decide whether Trusopt can be used.
Use in Patients with Renal or Hepatic Impairment
Inform the doctor about past and current kidney or liver diseases.
Driving and Operating Machinery
Some side effects (such as dizziness and blurred vision) may affect the ability to drive or operate machinery (see section 4. Possible side effects). Until these symptoms have completely resolved, the patient should not drive or operate machinery.
Trusopt Contains Benzalkonium Chloride
This medication contains approximately 0.002 mg of benzalkonium chloride per drop, which corresponds to 0.075 mg/ml. Benzalkonium chloride may be absorbed by soft contact lenses and change their color. Remove contact lenses before administering the medication and wait at least 15 minutes before putting them back on. Benzalkonium chloride may also cause eye irritation, especially in patients with dry eye syndrome or corneal disorders (the transparent layer on the front of the eye). If abnormal sensations in the eye, stinging, or eye pain occur after using the medication, seek medical attention.
3. How to Use Trusopt
This medication should always be used as directed by a doctor. In case of doubts, consult a doctor or pharmacist. The doctor determines the dose and duration of treatment. If Trusopt is the only eye medication used, it is recommended to administer one drop into the affected eye(s) three times a day (morning, afternoon, and evening). If the doctor has also prescribed a beta-adrenergic blocker eye drop to reduce intraocular pressure, the recommended dose of Trusopt is one drop into the affected eye(s) twice a day - morning and evening. If another eye medication is used in addition to Trusopt, wait at least 15 minutes between administering the medications. Eye ointments should always be applied last. Do not change the dosage without consulting a doctor. If treatment needs to be discontinued, seek medical attention immediately. Do not touch the dropper tip to the eye or its surroundings. To avoid possible contamination of the solution, avoid contact between the dropper and any surface.
Instructions for Use:
Do not use if the plastic security strip around the neck of the container is damaged or missing. When opening the container for the first time, tear off the plastic security strip. When using the Trusopt medication:
- 1. Wash your hands
- 2. Open the container.
Be Particularly Careful Not to Touch the Eye Dropper to the Eye, Skin Around the Eyes
or Fingers.Failure to follow this recommendation may result in contamination of the eye drops. Using contaminated eye drops can lead to dangerous complications and even vision loss
- 3. Tilt your head back and hold the inverted container over the eye.
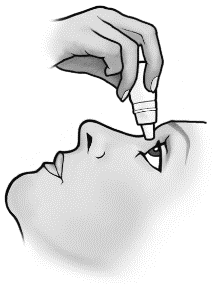
- 4. Pull the lower eyelid down and look up. Gently squeeze the container to release a single drop into the space between the lower eyelid and the eye.
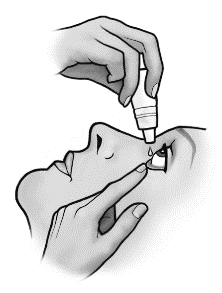
- 5. Close your eye and press the inner corner of the eye with your finger for about two minutes. This helps prevent the medication from entering the entire body.
- 6. To administer the medication to the second eye, if directed by a doctor, repeat steps 3 to 5.
- 7. Put the cap back on and tightly close the container.
Using More Than the Recommended Dose of Trusopt
In case of swallowing the contents of the container, seek medical attention immediately.
Missing a Dose of Trusopt
Trusopt should be used as directed by a doctor. If a dose is missed, administer it as soon as possible. However, if it is almost time for the next dose, do not administer the missed dose, and return to the previously established dosing schedule.
Stopping the Use of Trusopt
Before discontinuing the medication, consult a doctor. If there are any further doubts about using this medication, consult a doctor or pharmacist.
4. Possible Side Effects
Like all medications, Trusopt can cause side effects, although not everybody gets them. In clinical trials, the most common side effects (occurring in about 3% of patients) were eye disorders, mainly conjunctivitis and eyelid reactions. If the patient experiences an allergic reaction, including hives, face, lip, tongue, and/or throat swelling, which may cause difficulty breathing or swallowing, they should stop using the medication and seek medical attention immediately. Possible side effects reported during clinical trials or after the medication was marketed, occurring in patients treated with Trusopt, are listed below according to their frequency.
Very Common (occurring in more than 1 in 10 people):
- eye burning and stinging
Common (occurring in 1 to 10 in 100 patients):
- headache
- superficial punctate keratitis, tearing, conjunctivitis, blepharitis, eye itching, eyelid irritation, blurred vision
- nausea, bitter taste in the mouth
- feeling of weakness and fatigue
Uncommon (occurring in 1 to 10 in 1000 patients):
- uveitis and iritis
Rare (occurring in 1 to 10 in 10,000 patients):
- dizziness, feeling of tingling and numbness
- irritation, including redness, pain, eyelid sticking, transient myopia (resolving after discontinuation of therapy), corneal edema after filtration surgery
- epistaxis
- throat irritation, dry mouth
- contact dermatitis, severe skin reactions (Stevens-Johnson syndrome, toxic epidermal necrolysis)
- urolithiasis
- subjective and objective symptoms of local reactions (eyelid reactions) and symptoms of general allergic reactions, including angioedema, urticaria, and pruritus, rash, bronchospasm
Frequency Not Known (frequency cannot be estimated from the available data)
- dyspnea
- feeling of a foreign body in the eye (feeling that something is in the eye)
- palpitations, which may be rapid or irregular (heart pounding)
- tachycardia
- increased blood pressure
Reporting Side Effects
If any side effects occur, including any side effects not listed in this leaflet, inform a doctor or pharmacist. Side effects can be reported directly to the Department of Drug Safety Monitoring of the Office for Registration of Medicinal Products, Medical Devices, and Biocidal Products: Al. Jerozolimskie 181C, 02-222 Warsaw, Tel.: +48 22 49 21 301, Fax: +48 22 49 21 309, website: https://smz.ezdrowie.gov.pl. Side effects can also be reported to the marketing authorization holder. By reporting side effects, more information can be collected on the safety of the medication.
5. How to Store Trusopt
Store the medication out of sight and reach of children. Store in a temperature below 25°C, in the original outer packaging. Protect from light. Do not use this medication after the expiration date stated on the packaging after "EXP". The expiration date refers to the last day of the specified month. Shelf life after opening the container: 4 weeks. Medications should not be disposed of via wastewater or household waste containers. Ask a pharmacist how to dispose of unused medications. This will help protect the environment.
6. Contents of the Package and Other Information
What Trusopt Contains
- The active substance of Trusopt is dorzolamide. 1 ml of solution contains 20 mg of dorzolamide (as dorzolamide hydrochloride)
- The other ingredients are: benzalkonium chloride, hydroxyethylcellulose, mannitol, sodium citrate, sodium hydroxide (to adjust the pH), and water for injections.
What Trusopt Looks Like and What the Package Contains
Trusopt is a clear, colorless or almost colorless, slightly viscous solution. Trusopt is available in a white, semi-transparent plastic container containing 5 ml of solution. The plastic container is closed with a white cap. The unbroken security strip on the container label indicates that the product has not been used. Packaging: 1 x 5 ml (1 container of 5 ml), 3 x 5 ml (tripack containing 3 containers of 5 ml). Not all pack sizes may be marketed.
Marketing Authorization Holder and Manufacturer
Marketing Authorization Holder
Santen Oy, Niittyhaankatu 20, 33720 Tampere, Finland
Manufacturer
Santen Oy, Kelloportinkatu 1, 33100 Tampere, Finland
Date of Last Revision of the Package Leaflet: 02/2023
- Country of registration
- Active substance
- Prescription requiredYes
- ImporterSanten OY
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to TrusoptDosage form: Drops, 20 mg/mlActive substance: dorzolamideManufacturer: Bruschettini S.r.I.Prescription requiredDosage form: Drops, 20 mg/mlActive substance: dorzolamidePrescription requiredDosage form: Drops, 20 mg/mlActive substance: dorzolamideManufacturer: Rafarm S.A.Prescription required
Alternatives to Trusopt in other countries
The best alternatives with the same active ingredient and therapeutic effect.
Alternative to Trusopt in Spain
Alternative to Trusopt in Ukraine
Online doctors for Trusopt
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for Trusopt – subject to medical assessment and local rules.














