
DORZOLAMIDE FARMALIDER 20 mg/ml EYE DROPS SOLUTION

How to use DORZOLAMIDE FARMALIDER 20 mg/ml EYE DROPS SOLUTION
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
PACKAGE LEAFLET: INFORMATION FOR THE USER
Dorzolamida Farmalider 20 mg/ml eye drops, solution
Dorzolamida
Read the package leaflet carefully before you start using this medicine because it contains important information for you.
- Keep this package leaflet, you may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the package leaflet
- What is Dorzolamida Farmalider and what is it used for
- What you need to know before you use Dorzolamida Farmalider
- How to use Dorzolamida Farmalider
- Possible side effects
- Storage of Dorzolamida Farmalider
- Contents of the pack and further information
1. What is DORZOLAMIDA Farmalider and what is it used for
This medicine contains dorzolamida, which belongs to a group of medicines called "carbonic anhydrase inhibitors". This medicine is prescribed to reduce high pressure in the eye and for the treatment of glaucoma. This medicine can be used alone or together with other medicines that reduce pressure in the eye (called beta-blockers).
2. What you need to know before you use Dorzolamida Farmalider
Do not use Dorzolamida Farmalider:
- if you are allergic to dorzolamida hydrochloride or any of the other ingredients of this medicine (listed in section 6).
- if you have kidney failure or severe kidney problems, or a history of kidney stones.
Warnings and precautions
Consult your doctor or pharmacist before starting to use this medicine.
Tell your doctor or pharmacist about any disease you have now or have had in the past, including eye problems and eye surgery, and about any allergy to medicines.
If you experience eye irritation or any new eye problem, such as redness of the eye or inflammation of the eyelids, contact your doctor immediately.
If you suspect that this medicine is causing an allergic reaction (such as a skin rash, severe skin reaction, or itching), stop using it and contact your doctor immediately.
Children
Dorzolamida has been studied in infants and children under 6 years of age with high eye pressure or with a diagnosis of glaucoma. For more information, consult your doctor.
Elderly
In studies with dorzolamida, the effects of this medicine were similar in elderly patients and younger patients.
Patients with liver failure
Tell your doctor about any liver problems you have now or have had in the past.
Using Dorzolamida Farmalider with other medicines
Tell your doctor or pharmacist if you are using, have recently used, or might use any other medicine (including eye drops), especially if you are using another carbonic anhydrase inhibitor, such as acetazolamide, or a medicine from the group of sulfonamides.
Pregnancy and breastfeeding
If you are pregnant or breastfeeding, think you may be pregnant, or plan to become pregnant, consult your doctor or pharmacist before using this medicine.
Use during pregnancy
This medicine should not be used during pregnancy. Tell your doctor if you are pregnant or plan to become pregnant.
Use during breastfeeding
If treatment with this medicine is necessary, breastfeeding is not recommended. Tell your doctor if you are breastfeeding or plan to breastfeed.
Driving and using machines
No studies have been conducted on the effects on the ability to drive or use machines. There are some side effects associated with this medicine, such as dizziness and blurred vision, that may affect your ability to drive and/or use machines. Do not drive or operate tools or machines until you feel well or your vision has cleared.
Dorzolamida contains benzalkonium chloride
This medicine contains 0.075 mg/ml of benzalkonium chloride as a preservative. Benzalkonium chloride may be absorbed by soft contact lenses and may change the color of the contact lenses. You should remove your contact lenses before using this medicine and wait at least 15 minutes before putting them back.
Benzalkonium chloride may also cause eye irritation, especially if you have dry eyes or corneal disorders (the transparent layer on the front of the eye). If you feel any abnormal sensation in the eyes, itching, or pain in the eye after using this medicine, talk to your doctor.
3. How to use Dorzolamida Farmalider
Follow the instructions for administration of this medicine exactly as indicated by your doctor. If you are unsure, consult your doctor or pharmacist again. Your doctor will determine the correct dose and duration of treatment.
When this medicine is used alone, the recommended dose is one drop in the affected eye(s) in the morning, afternoon, and evening.
If your doctor has recommended using this medicine with a beta-blocker eye drop to reduce pressure in the eye, the recommended dose is one drop of Dorzolamida Farmalider in the affected eye(s) in the morning and evening.
If you use this medicine with another eye drop, the application of the drops should be spaced at least 10 minutes apart.
Do not let the tip of the dropper touch the eye or the surrounding areas. It could become contaminated with bacteria that can cause eye infections, leading to serious eye damage or even loss of vision.
To avoid possible contamination, wash your hands before using this medicine and keep the tip of the dropper away from contact with any surface. If you think the medicine may have become contaminated, or if you have an eye infection, consult your doctor immediately to determine if you should continue using this bottle.
Instructions for use:
- First, wash your hands.
- Avoid touching the eye (or any other surface) with the tip of the dropper.
- If you use soft contact lenses, you should remove them before using the drops and wait at least 15 minutes before putting them back.
- The drops come in a plastic bottle with a screw cap and a dust cap with a security seal. When using the bottle for the first time, remove the dust cap by twisting it counterclockwise to break the seal.
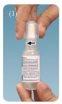
- Unscrew the inner cap
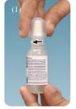
- Tilt your head back and look up.
- Gently pull down the lower eyelid to form a small pocket between the eyelid and your eye.
- Hold the bottle upside down over the eye and squeeze it gently to release one drop. DO NOT TOUCH THE EYE OR EYELID WITH THE TIP OF THE DROPPER.
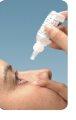
- Keep your eye closed and press the inner corner of your eye with the tip of your finger for two minutes. This helps prevent the medicine from reaching the rest of the body.
- Repeat in the other eye if your doctor has told you to do so.
- Replace the cap on the bottle after each use and tighten the cap over the dropper.
If you use more Dorzolamida Farmalider than you should
If you put too many drops in your eye or swallow the contents of the container, consult your doctor immediately.
In case of overdose or accidental ingestion, consult your doctor or pharmacist immediately or call the Toxicology Information Service, phone: 91 562 04 20, indicating the medicine and the amount ingested.
If you forget to use Dorzolamida Farmalider
It is important to use this medicine as directed by your doctor. If you miss a dose, use it as soon as possible. However, if it is almost time for your next dose, skip the missed dose and return to your regular dosing schedule.
Do not use a double dose to make up for the missed dose.
If you stop using Dorzolamida Farmalider
If you want to stop using this medicine, talk to your doctor first. If you have any further questions about the use of this medicine, ask your doctor or pharmacist.
4. Possible side effects
Like all medicines, Dorzolamida can cause side effects, although not everybody gets them.
If you experience allergic reactions, including hives, swelling of the face, lips, tongue, and/or throat that may cause difficulty breathing or swallowing, stop using this medicine and consult your doctor immediately.
The following side effects have been reported with the use of Dorzolamida during clinical trials or post-marketing use:
Very common: (may affect more than 1 in 10 people)
Burning and stinging in the eyes
Common: (may affect up to 1 in 10 people)
Corneal disease with eye pain and blurred vision (superficial punctate keratitis), discharge with itching of the eyes (conjunctivitis), irritation/inflammation of the eyelid, blurred vision, headache, nausea, bitter taste, and fatigue.
Uncommon: (may affect up to 1 in 100 people)
Iritis (inflammation of the iris).
Rare: (may affect up to 1 in 1,000 people)
Numbness or tingling of hands and feet, temporary myopia that may resolve with discontinuation of treatment, fluid accumulation under the retina (choroidal detachment after filtration surgery), eye pain, crust formation on the eyelid, low eye pressure, corneal inflammation (with symptoms of visual disturbances), eye irritation with redness, kidney stones, dizziness, nosebleeds, throat irritation, dry mouth, localized skin rash (contact dermatitis), severe skin reactions, allergic reactions such as skin rash, hives, itching, and in rare cases, possible swelling of the lips, eyes, and mouth, difficulty breathing, and more rarely, wheezing.
Frequency not known(frequency cannot be estimated from the available data)
Difficulty breathing
Foreign body sensation in the eye (feeling of having something in the eye) and strong or irregular heartbeats (palpitations).
Increased heart rate.
Increased blood pressure.
Reporting of side effects:
If you experience any side effects, talk to your doctor, pharmacist, or nurse, even if it is possible side effects not listed in this leaflet. You can also report side effects directly through the Spanish Pharmacovigilance System for Human Use Medicines: https://www.notificaram.es. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of DORZOLAMIDA Farmalider
Keep this medicine out of the sight and reach of children.
Do not store above 25°C. Keep the bottle in the outer packaging to protect it from light.
Use the solution within 28 days of opening the bottle.
Do not use Dorzolamida after the expiry date stated on the bottle and carton.
Medicines should not be disposed of via wastewater or household waste. Return the containers and any unused medicine to a pharmacy for disposal. If you are unsure, ask your pharmacist how to dispose of the containers and any unused medicine. This will help protect the environment.
6. Contents of the pack and further information
Composition of Dorzolamida Farmalider
- The active substance is dorzolamida.
- Each ml contains 22.26 mg of dorzolamida hydrochloride, equivalent to 20 mg of dorzolamida.
- The other ingredients are hydroxyethylcellulose, mannitol, sodium citrate, sodium hydroxide, and water for injections. Benzalkonium chloride is added as a preservative.
Appearance of the product and pack contents
A bottle of Dorzolamida contains 5 ml of solution. Dorzolamida is a clear, colorless, and slightly viscous solution, packaged in a 5 ml low-density polyethylene (LDPE) bottle, labeled, with an orange screw cap and a dust cap with a security seal that seals the bottle cap.
Marketing authorization holder and manufacturer
Marketing authorization holder
Farmalider, S.A.
C/ La Granja, 1
28108 Alcobendas (Madrid)
Madrid
Manufacturer
FDC Pharma
Unit 6, Fulcrum 1, Solent Way, Whiteley, Fareham, Hampshire, PO15 7FE
United Kingdom
Phone: +44 (0) 1489 565222
Fax: +44 (0) 1489 565222
Email: [email protected]
Date of last revision of this leaflet: December 2022.
Detailed and updated information on this medicine is available on the website of the Spanish Agency for Medicines and Health Products (AEMPS) http://www.aemps.gob.es/
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to DORZOLAMIDE FARMALIDER 20 mg/ml EYE DROPS SOLUTIONDosage form: EYE DROP, 20 mg/mlActive substance: dorzolamideManufacturer: Tiedra Farmaceutica S.L.Prescription requiredDosage form: EYEDROP, 20 mg/mlActive substance: dorzolamideManufacturer: Brill Pharma S.L.Prescription requiredDosage form: EYE DROP, 2%Active substance: dorzolamideManufacturer: Aristo Pharma Iberia S.L.Prescription required
Online doctors for DORZOLAMIDE FARMALIDER 20 mg/ml EYE DROPS SOLUTION
Discuss questions about DORZOLAMIDE FARMALIDER 20 mg/ml EYE DROPS SOLUTION, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions















