TRUSOPT 20 mg/ml EYE DROPS SOLUTION

How to use TRUSOPT 20 mg/ml EYE DROPS SOLUTION
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the User
TRUSOPT 20 mg/ml eye drops, solution
dorzolamida
Read this package leaflet carefully before you start using this medicine because it contains important information for you.
- Keep this package leaflet, you may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the package leaflet
- What is TRUSOPT and what is it used for
- What you need to know before you use TRUSOPT
- How to use TRUSOPT
- Possible side effects
- Storage of TRUSOPT
- Contents of the pack and further information
1. What is TRUSOPT and what is it used for
TRUSOPT contains dorzolamida, which belongs to a group of medicines called "carbonic anhydrase inhibitors".
This medicine is prescribed to lower high pressure in the eye and to treat glaucoma. This medicine may be used alone or in combination with other medicines that lower pressure in the eye (called beta-blockers).
2. What you need to know before you use TRUSOPT
Do not use TRUSOPT
- if you are allergic to dorzolamida hydrochloride or any of the other ingredients of this medicine (listed in section 6).
- if you have severe kidney problems or a history of kidney stones.
Warnings and precautions
Consult your doctor or pharmacist before you start using TRUSOPT.
Tell your doctor or pharmacist if you have or have had any medical problems, including eye problems and eye surgeries, and if you have any allergy to any medication.
If you experience any eye irritation or new eye disorder, such as redness of the eye or swelling of the eyelids, consult your doctor immediately.
If you suspect that TRUSOPT is causing an allergic reaction (for example, skin rash, severe skin reaction, or itching), stop using this medicine and consult your doctor immediately.
Children
TRUSOPT has been studied in infants and children under 6 years of age who had high pressure in the eye(s) or had been diagnosed with glaucoma. For more information, consult your doctor.
Older people
In studies with TRUSOPT, the effects of this medicine were similar in both older and younger patients.
Use in patients with liver impairment
Tell your doctor if you have or have had liver problems.
Using TRUSOPT with other medicines
Tell your doctor or pharmacist if you are using, have recently used, or might use any other medicines (including eye drops). This is especially important if you are taking another carbonic anhydrase inhibitor such as acetazolamide, or a sulphonamide.
Pregnancy and breastfeeding
If you are pregnant or breastfeeding, think you may be pregnant, or are planning to have a baby, ask your doctor or pharmacist for advice before using this medicine.
Pregnancy
Do not use this medicine during pregnancy. Tell your doctor if you are pregnant or planning to become pregnant.
Breastfeeding
If treatment with this medicine is required, breastfeeding is not recommended. Tell your doctor if you are breastfeeding or planning to breastfeed.
Driving and using machines
No studies on the effects on the ability to drive and use machines have been performed. There are side effects associated with TRUSOPT, such as dizziness and blurred vision, that may affect your ability to drive and/or operate machinery. Do not drive or operate machinery until you feel well or your vision is clear.
TRUSOPT contains benzalkonium chloride
This medicine contains approximately 0.002 mg of benzalkonium chloride in each drop, equivalent to 0.075 mg/ml.
Benzalkonium chloride may be absorbed by soft contact lenses, altering their colour. Remove contact lenses before using this medicine and wait 15 minutes before putting them back.
Benzalkonium chloride can cause eye irritation, especially if you have dry eyes or other corneal diseases (the transparent layer on the front of the eye). Consult your doctor if you feel any unusual sensation, itching, or pain in the eye after using this medicine.
3. How to use TRUSOPT
Follow exactly the instructions for administration of this medicine given by your doctor. If you are unsure, consult your doctor or pharmacist again. The appropriate dosage and duration of treatment will be determined by your doctor.
When this medicine is the only medicine used, the recommended dose is one drop in the affected eye(s) in the morning, afternoon, and evening.
If your doctor has recommended that you use this medicine with a beta-blocker eye drop to reduce eye pressure, the recommended dose is one drop of TRUSOPT in the affected eye(s) in the morning and evening.
If you use TRUSOPT with another eye drop, the drops should be instilled with a minimum time interval of 10 minutes.
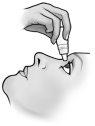
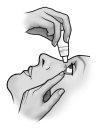
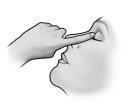
Do not let the tip of the container touch the eyes or the areas surrounding them. It may be contaminated with bacteria that can cause eye infections leading to serious damage to the eyes, including loss of vision. To avoid possible contamination of the container, wash your hands before using this medicine and avoid touching the tip of the container to any surface. If you think your medicine may be contaminated, or if you develop an eye infection, consult your doctor immediately if you can continue using this container.
Instructions for use
Do not use the container if the plastic safety strip around the neck of the container is missing or broken. When you open the container for the first time, tear off the plastic safety strip.
Each time you use TRUSOPT:
| |
|
|
|
|
|
|
If you use more TRUSOPT than you should
If you put too many drops in your eye or swallow some of the contents of the container, you should tell your doctor immediately.
In case of overdose or accidental ingestion, consult your doctor or pharmacist immediately or call the Toxicology Information Service, telephone (91) 562 04 20, stating the medicine and the amount taken.
If you forget to use TRUSOPT
It is important to use this medicine as your doctor has told you. If you forget to use a dose, use it as soon as you remember. However, if it is almost time for your next dose, do not use the missed dose. Continue with your normal dosing schedule.
Do not use a double dose to make up for forgotten doses.
If you stop using TRUSOPT
If you want to stop using this medicine, consult your doctor first. If you have any further questions on the use of this medicine, ask your doctor or pharmacist.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
If you develop allergic reactions such as hives, swelling of the face, lips, tongue, and/or throat that may cause difficulty in breathing or swallowing, stop using this medicine and consult your doctor immediately.
The following side effects have been reported with TRUSOPT during clinical trials or after marketing:
Very common side effects:(may affect more than 1 in 10 people)
Burning and stinging of the eyes.
Common side effects:(may affect up to 1 in 10 people)
Corneal disease with eye pain and blurred vision (superficial punctate keratitis), discharge with itching of the eyes (conjunctivitis), irritation/inflammation of the eyelid, blurred vision, headache, nausea, bitter taste, and fatigue.
Uncommon side effects:(may affect up to 1 in 100 people)
Inflammation of the iris.
Rare side effects:(may affect up to 1 in 1,000 people)
Numbness or tingling of the hands or feet, transient myopia that resolves when therapy is stopped, development of fluid under the retina (choroidal detachment, after filtration surgery), eye pain, crusts on the eyelid, low eye pressure, inflammation of the cornea (with symptoms of visual disturbances), eye irritation including redness, kidney stones, dizziness, nosebleeds, throat irritation, dry mouth, localized skin rash (contact dermatitis), severe skin reactions, allergic reactions such as skin rash, hives, itching, and in rare cases swelling of the lips, eyes, and mouth, shortness of breath, and more rarely difficulty breathing.
Frequency not known:(cannot be estimated from the available data)
Difficulty breathing, feeling of a foreign body in the eye (feeling of having something in the eye), strong and/or irregular heartbeats (palpitations), increased heart rate, and increased blood pressure.
Reporting of side effects
If you experience any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. You can also report side effects directly to the Spanish Medicines Agency's website: https://www.notificaram.es. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of TRUSOPT
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date which is stated on the label of the container and the carton after 'EXP'. The expiry date is the last day of the month stated.
TRUSOPT should be used within 28 days of opening the container.
This medicine does not require any special storage conditions. Keep the container in the outer carton to protect it from light.
Medicines should not be disposed of via wastewater or household waste. Return the containers and any unused medicine to a pharmacy for disposal. If you are unsure, ask your pharmacist how to dispose of the containers and any unused medicine. This will help protect the environment.
6. Contents of the pack and further information
Composition of TRUSOPT
- The active substance is dorzolamida.
- Each ml contains 22.26 mg of dorzolamida hydrochloride equivalent to 20 mg of dorzolamida.
- The other ingredients are hydroxyethylcellulose, mannitol, sodium citrate, sodium hydroxide, and water for injections. Benzalkonium chloride is added as a preservative.
Appearance and packaging of the product
TRUSOPT is a clear, colourless to almost colourless, slightly viscous solution.
TRUSOPT is available in a 5 ml solution in a white translucent container. The plastic container is closed with a white screw cap.
The safety strip on the label of the container provides evidence that the medicine has not been tampered with.
Container sizes:
1 x 5 ml (one 5 ml container)
3 x 5 ml (three 5 ml containers)
6 x 5 ml (six 5 ml containers)
Not all container sizes may be marketed.
Marketing authorisation holder and manufacturer
Marketing authorisation holder
Santen Oy
Niittyhaankatu 20
33720 Tampere
Finland
Manufacturer
Santen Oy
Kelloportinkatu 1
33100 Tampere
Finland
Local representative
Santen Pharmaceutical Spain S.L.
Acanto, 22, 7º
28045 – Madrid
This medicine is authorised in the Member States of the European Economic Area under the following names:
Austria, Belgium, Denmark, Finland, France, Germany, Greece, Ireland, Italy, Netherlands, Portugal, Spain, and Sweden:
TRUSOPT
Date of last revision of this leaflet:06/2023
Detailed and up-to-date information on this medicine is available on the website of the Spanish Agency for Medicines and Health Products (AEMPS) http://www.aemps.gob.es/
- Country of registration
- Average pharmacy price5.12 EUR
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to TRUSOPT 20 mg/ml EYE DROPS SOLUTIONDosage form: EYE DROP, 20 mg/mlActive substance: dorzolamideManufacturer: Tiedra Farmaceutica S.L.Prescription requiredDosage form: EYEDROP, 20 mg/mlActive substance: dorzolamideManufacturer: Brill Pharma S.L.Prescription requiredDosage form: EYE DROP, 2%Active substance: dorzolamideManufacturer: Aristo Pharma Iberia S.L.Prescription required
Online doctors for TRUSOPT 20 mg/ml EYE DROPS SOLUTION
Discuss questions about TRUSOPT 20 mg/ml EYE DROPS SOLUTION, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions