
ARZOLAN 20 mg/ml EYE DROPS SOLUTION
Ask a doctor about a prescription for ARZOLAN 20 mg/ml EYE DROPS SOLUTION

How to use ARZOLAN 20 mg/ml EYE DROPS SOLUTION
Introduction
Package Leaflet: Information for the User
Arzolan 20 mg/ml eye drops, solution
Dorzolamida
Read this package leaflet carefully before you start using this medicine because it contains important information for you.
- Keep this package leaflet, you may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this package leaflet.
Contents of the package leaflet
- What is Arzolan and what is it used for
- What you need to know before you use Arzolan
- How to use Arzolan
- Possible side effects
- Storage of Arzolan
- Contents of the pack and further information
1. What is Arzolan and what is it used for
Arzolan is a sterile eye drop solution. Arzolan contains the active substance dorzolamida, a compound related to sulfonamides.
Dorzolamida is a carbonic anhydrase inhibitor for ophthalmic use and reduces high pressure inside the eye.
It is indicated for the treatment of high intraocular pressure in patients with ocular hypertension and glaucoma (open-angle glaucoma, pseudoexfoliative glaucoma). Arzolan can be used alone or in combination with other medications that decrease pressure inside the eye (called beta-blockers).
2. Arzolan
Do not use Arzolan
- If you are allergic (hypersensitive) to dorzolamida or any of the other ingredients of this solution.
- If you have severe kidney problems.
- If you have a pH disorder (acid-base balance) in the blood.
Warnings and precautions
Before starting treatment with Arzolan, consult your doctor:
- If you have or have had liver problems in the past
- If you have been informed that you have a corneal defect
- If you have had an allergy to any medication
- If you have undergone or are going to undergo eye surgery
- If you have suffered an eye injury or have an eye infection
- If you have a history of kidney stones
- If you are taking another carbonic anhydrase inhibitor
- If you use contact lenses (see the section "Important information about the ingredients of Arzolan").
- If you experience any eye irritation or new eye disorder, such as eye redness or eyelid swelling, consult your doctor immediately.
- If you suspect that Arzolan is causing an allergic reaction (e.g., skin rash or itching, eye inflammation), stop using it and consult your doctor as soon as possible.
Children and adolescents
Arzolan should only be used in children if the benefits justify the risks. Your doctor will advise you.
Using Arzolan with other medicines
Tell your doctor or pharmacist if you are using or have recently used other medicines, including those obtained without a prescription.
In particular, you should consult your doctor if you are using another carbonic anhydrase inhibitor such as acetazolamide. You may be using this type of medicine orally, as eye drops, or in any other way.
Pregnancy and breastfeeding
Consult your doctor or pharmacist before using any medicine. Inform your doctor if you are pregnant or plan to become pregnant. You should not use Arzolan during pregnancy unless your doctor recommends it.
You should not use Arzolan during breastfeeding.
Driving and using machines
Arzolan may cause dizziness and visual disturbances in some patients. Do not drive or operate machinery until the symptoms have disappeared.
Arzolan contains benzalkonium chloride
This medicine may cause eye irritation because it contains benzalkonium chloride.
Avoid contact with soft contact lenses.
Remove contact lenses before application and wait at least 15 minutes before putting them back.
It changes the color of soft contact lenses.
3. How to use Arzolan
Follow the instructions for using Arzolan exactly as indicated by your doctor. Consult your doctor or pharmacist if you have any doubts.
The appropriate dosage and duration of treatment will be determined by your doctor.
When Arzolan is used alone, the usual dose is one drop in the affected eye(s) three times a day, i.e., in the morning, afternoon, and evening.
If your doctor has recommended using Arzolan with a beta-blocker eye drop (medicines that decrease the pressure inside the eye), the usual dose is one drop in the eye(s) twice a day, for example, in the morning and afternoon.
If you are going to use Arzolan with another eye drop, the applications of the different medicines should be spaced at least 10 minutes apart. Similarly, when you are going to replace another eye drop medicine with Arzolan, used to decrease eye pressure, you should stop using the other medicine after taking the appropriate dose for one day and start using Arzolan the next day.
Do not change the dose of the medicine without consulting your doctor. If you need to interrupt treatment, consult your doctor immediately.
Do not let the tip of the container touch the eyes or the surrounding areas. It may be contaminated with bacteria that can cause eye infections that can lead to serious damage to the eyes, including vision loss. To avoid possible contamination of the container, avoid letting the tip of the container come into contact with any surface.
Instructions for use:
It is recommended to wash your hands before applying the eye drops.
Sit down, if possible, in front of a mirror to facilitate application.
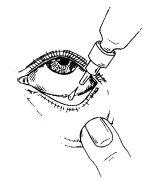
- Before using the medicine for the first time, make sure the security strip on the neck of the bottle is intact. When the bottle has not been opened yet, it is normal to have a space between the bottle and the cap.
- Remove the cap from the bottle.
- Tilt your head back and gently separate the lower eyelid, forming a small gap between the eyelid and the eye.
- Invert the bottle and press until a single drop is dispensed into the eye according to your doctor's instructions. DO NOT TOUCH THE EYE OR EYELID WITH THE DROPPER TIP.
- Close your eye and press the inner corner of the eye with your finger for about two minutes. This helps prevent the medicine from reaching the rest of the body.
- Repeat steps 3 and 4 in the other eye if your doctor has indicated it.
- Replace the cap and close the bottle immediately after use.
If you use more Arzolan than you should
If you apply too many drops in the eye or swallow part of the contents of the bottle, consult your doctor immediately.
In case of overdose or accidental ingestion, consult your doctor or pharmacist immediately or call the Toxicology Information Service, phone: 91 562 04 20.
If you forget to use Arzolan
It is important to administer Arzolan as your doctor has indicated.
If you forget to apply a dose, you should administer it as soon as possible. However, if it is almost time for the next dose, do not administer the missed dose and continue with the normal dosing schedule.
Do not use a double dose to make up for missed doses.
If you stop using Arzolan
Arzolan should be used daily to have the desired effect. If you need to interrupt treatment, consult your doctor immediately.
If you have any further questions about the use of this product, ask your doctor or pharmacist.
4. Possible side effects
Like all medicines, Arzolan can cause side effects, although not everybody gets them.
The following terms are used to describe how often side effects are reported:
Very common | May affect more than 1 in 10 patients |
Common | May affect up to 1 in 100 patients |
Uncommon | May affect up to 1 in 1,000 patients |
Rare | May affect up to 1 in 10,000 patients |
Frequency not known | Cannot be estimated from the available data |
You may experience any of the following side effects with Arzolan
Ocular disorders:
Very common: burning and stinging
Common: inflammation or swelling of the surface of the eye(s) and possible inflammation of the eyelid(s) and/or around the eye(s), tearing or itching, blurred vision, effects on the surface of the eye
Uncommon: inflammation of the middle layer of the eyeball
Rare: swelling of the surface of the eye(s), choroidal detachment with symptoms of visual disturbances (after filtration surgery), ocular hypotony, eye redness, eye pain, eyelid crusts, transient myopia (which resolves after therapy is stopped)
Gastrointestinal disorders:
Common: nausea, bitter taste
Rare: throat irritation, dry mouth
General disorders and administration site conditions:
Common: asthenia/fatigue
Rare: Hypersensitivity: signs and symptoms of local reactions (eyelid reactions) and systemic allergic reactions including swelling of the face, lips, tongue, and/or throat that can cause difficulty breathing or swallowing, urticaria, and itching, skin rash, shortness of breath, and rarely bronchospasm (contraction of smooth bronchial muscle)
Nervous system disorders:
Common: headache
Rare: dizziness, numbness/tingling
Renal and urinary disorders:
Rare: formation of kidney stones
Respiratory, thoracic, and mediastinal disorders:
Rare: nasal bleeding
Skin and subcutaneous tissue disorders:
Rare: skin inflammation
Cardiac disorders:
Frequency not known: strong heartbeats that can be rapid or irregular (palpitations), increased heart rate
Vascular disorders:
Frequency not known: increased blood pressure
Reporting of side effects
If you experience any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this package leaflet. You can also report side effects directly through the Spanish Medicines Monitoring System: www.notificaRAM.es. By reporting side effects, you can help provide more information on the safety of this medicine.
If you consider that any of the side effects you are experiencing is serious or if you notice any side effect not mentioned in this package leaflet, inform your doctor or pharmacist.
5. Storage of Arzolan
Keep out of sight and reach of children.
Do not use Arzolan after the expiration date stated on the packaging after EXP. The expiration date is the last day of the month indicated.
Store the bottle in the outer packaging to protect it from light.
Store below 30°C.
Arzolan should be used within 28 days of first opening the bottle. Therefore, you should discard the bottle 4 weeks after first opening it, even if there is still solution left. As a reminder, write the opening date of each bottle on the space on the carton.
Medicines should not be disposed of via wastewater or household waste. Dispose of the packaging and any unused medicine in the pharmacy's SIGRE collection point. If you have any doubts, ask your pharmacist how to dispose of the packaging and any unused medicine. This will help protect the environment.
6. Arzolan
Composition of Arzolan
- The active substance is dorzolamida. Each ml contains 20 mg of dorzolamida (equivalent to dorzolamida hydrochloride).
- The other ingredients are mannitol, hydroxyethyl cellulose, benzalkonium chloride (as a preservative), sodium citrate, sodium hydroxide to adjust the pH, and water for injectable preparations.
Appearance of the product and packaging contents
Arzolan is a sterile, isotonic, buffered, colorless, slightly viscous solution in a low-density polyethylene bottle with a sealed dropper and a two-piece cap. Each bottle contains 5 ml of the eye drop solution.
Arzolan is available in packs of 1 bottle with 5 ml of eye drop solution.
Marketing authorization holder and manufacturer
Marketing authorization holder:
Tiedra Farmacéutica, S.L.
C/ Colón, 7
30510 Yecla (Murcia)
Spain
Manufacturer:
Pharmathen S.A.
6, Dervenakion str.
153 51 Pallini Attiki,
Greece
Date of last revision of this package leaflet: January 2023
Detailed and updated information on this medicine is available on the website of the Spanish Agency for Medicines and Health Products (AEMPS) http://www.aemps.gob.es/

How much does ARZOLAN 20 mg/ml EYE DROPS SOLUTION cost in Spain ( 2026)?
The average price of ARZOLAN 20 mg/ml EYE DROPS SOLUTION in January, 2026 is around 5.12 EUR. Prices may vary depending on the region, pharmacy, and whether a prescription is required. Always check with a local pharmacy or online source for the most accurate information.
- Country of registration
- Average pharmacy price5.12 EUR
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to ARZOLAN 20 mg/ml EYE DROPS SOLUTIONDosage form: EYEDROP, 20 mg/mlActive substance: dorzolamideManufacturer: Brill Pharma S.L.Prescription requiredDosage form: EYE DROP, 2%Active substance: dorzolamideManufacturer: Aristo Pharma Iberia S.L.Prescription requiredDosage form: EYE DROP, 2%Active substance: dorzolamideManufacturer: Farmalider S.A.Prescription required
Alternatives to ARZOLAN 20 mg/ml EYE DROPS SOLUTION in other countries
The best alternatives with the same active ingredient and therapeutic effect.
Alternative to ARZOLAN 20 mg/ml EYE DROPS SOLUTION in Poland
Alternative to ARZOLAN 20 mg/ml EYE DROPS SOLUTION in Ukraine
Online doctors for ARZOLAN 20 mg/ml EYE DROPS SOLUTION
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for ARZOLAN 20 mg/ml EYE DROPS SOLUTION – subject to medical assessment and local rules.














