
How to use Treosulfan Zentiva
Leaflet attached to the packaging: information for the user
Treosulfan Zentiva, 5 g, powder for solution for infusion
Treosulfan
Read carefully the contents of the leaflet before using the medicine, as it contains important information for the patient.
- Keep this leaflet, you may need to read it again.
- In case of any doubts, consult a doctor or nurse.
- If the patient experiences any side effects, including any possible side effects not listed in the leaflet, tell your doctor or nurse. See section 4.
Table of contents of the leaflet
- 1.What is Treosulfan Zentiva and what is it used for
- 2.Important information before using Treosulfan Zentiva
- 3.How to use Treosulfan Zentiva
- 4.Possible side effects
- 5.How to store Treosulfan Zentiva
- 6.Contents of the pack and other information
1. What is Treosulfan Zentiva and what is it used for
Treosulfan Zentiva contains the active substance treosulfan. Treosulfan belongs to a group of anti-cancer medicines called alkylating agents. These medicines inhibit tumor growth.
Treosulfan Zentiva has been prescribed to the patient by a doctor for the treatment of advanced ovarian cancer after at least one previous standard therapy.
2. Important information before using Treosulfan Zentiva
When not to use Treosulfan Zentiva
- if the patient is allergic to treosulfan
- if the patient has too few blood cells (severe bone marrow depression). Before each administration, the patient will have a blood test to check if the number of blood cells allows the administration of Treosulfan Zentiva.
- during breastfeeding.
Warnings and precautions
Before starting Treosulfan Zentiva, discuss with your doctor or nurse if:
- the patient has pneumonia that causes shortness of breath (allergic pneumonia or pulmonary fibrosis). In such a case, Treosulfan Zentiva treatment should be discontinued. When using Treosulfan Zentiva, the patient should be aware of:
- increased risk of developing certain types of infections;
- the possibility of developing various blood cancers after long-term treatment;
- since treosulfan is excreted by the kidneys, blood morphology should be closely monitored and the dose modified if the patient develops kidney function disorders;
- cancer treatment may increase the risk of generalized infection after certain vaccinations. Therefore, this medicine should not be used with live vaccines;
- due to the possibility of bladder inflammation or frequent urination or a feeling of urgency to urinate with or without blood in the urine (hemorrhagic cystitis), it is recommended to drink more fluid than usual for up to 24 hours after treosulfan treatment; In the case of women of childbearing age during treatment and for six months after its completion, effective contraception (e.g. birth control pills) is also necessary (see section "Pregnancy and breastfeeding").
Treosulfan Zentiva and other medicines
Tell your doctor or nurse about all medicines the patient is taking or has recently taken, as well as any medicines the patient plans to take.
The effect of ibuprofen or chloroquine may be reduced when given in combination with Treosulfan Zentiva.
Pregnancy and breastfeeding
If the patient is pregnant or breastfeeding, thinks she may be pregnant or plans to have a child, she should consult a doctor before using this medicine. There are no data or only limited data on the use of Treosulfan Zentiva, 5-gram powder for solution for infusion in pregnant or breastfeeding women.
Pregnancy
Since fetal damage cannot be excluded, Treosulfan Zentiva, 5-gram powder for solution for infusion should not be used during pregnancy unless the doctor considers it absolutely necessary. The patient should not become pregnant while being treated with Treosulfan Zentiva, 5-gram powder for solution for infusion.
If the patient becomes pregnant during treatment with Treosulfan Zentiva, 5-gram powder for solution for infusion, she should inform her doctor immediately.
Contraception in women
For women of childbearing age during treatment with Treosulfan Zentiva, 5-gram powder for solution for infusion and for six months after its completion, effective contraception (e.g. birth control pills) is necessary.
Breastfeeding
Since it cannot be excluded that the substance may pass into breast milk, breastfeeding should not be done during treatment with Treosulfan Zentiva, 5-gram powder for solution for infusion.
Driving and using machines
In case of nausea or vomiting, there may be a decrease in the ability to drive vehicles and operate machines. If the patient experiences such symptoms, they should not drive vehicles or operate machines.
3. How to use Treosulfan Zentiva
Treosulfan Zentiva will be administered by a doctor or nurse in the form of an intravenous infusion. This will take from 15 to 30 minutes (intravenous infusion), and the medicine will be administered in a dose calculated specifically for the patient by the doctor.
The doctor will calculate the necessary dose of Treosulfan Zentiva based on the patient's blood morphology.
The doctor will reduce the dose if the patient has received another anti-cancer medicine or radiation therapy. The dose the patient receives also depends on the patient's body size and varies depending on the body surface area (BSA).
During treatment with Treosulfan Zentiva, infusions are usually given every 3 to 4 weeks. Basically, 6 treatment cycles are administered.
The doctor may change the dose and frequency of administration depending on the results of blood tests, the patient's overall health, other therapies used by the patient, and their response to treatment with Treosulfan Zentiva. In case of any doubts about treatment, consult a doctor or nurse.
If the patient experiences pain at the injection site, they should immediately inform their doctor or nurse.
Use in children
This medicine is not recommended for use in children.
Use of a higher than recommended dose of Treosulfan Zentiva
If the patient receives too much of this medicine, they may become ill or their blood cell count may decrease. The doctor may perform a blood transfusion or take other actions if necessary.
In case of any further doubts about the use of this medicine, consult a doctor or nurse.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them. The doctor will discuss them with the patient and explain the risks and benefits of treatment.
Report immediately to a doctor or nurse if any of the following symptoms occur:
- Allergic reactions [rare (may occur in less than 1 in 1000 patients)]: if the patient experiences itching, rash, swelling of the face, lips, tongue and/or throat, which may cause difficulty in swallowing or breathing or a drop in blood pressure.
- Fever or infection [very common (may occur in more than 1 in 10 patients)]: if the patient has a fever of 38°C or higher, sweating or symptoms of infection (since the patient may have a lower than usual number of white blood cells).
- Weakness [very common (may occur in more than 1 in 10 patients)]shortness of breath or paleness of the skin (since the patient may have a lower than usual number of red blood cells).
- Bleeding [very common (may occur in more than 1 in 10 patients)]from the gums, mouth or nose or unexpected bruising or paleness of the skin (since the patient may have a lower than usual number of platelets).
- Breathing difficulties [ very rare (may occur in less than 1 in 10,000 patients)](since the patient may experience an allergic reaction, inflammation or infection of the lungs).
Very common (may occur in more than 1 in 10 patients):
- Nausea, including nausea (nausea) with vomiting or without vomiting.
- Mild hair loss. After the procedure, hair growth should return to normal.
- Brown skin discoloration.
Common (may occur in less than 1 in 10 patients):
- Infections caused by bacteria, viruses or fungi.
Uncommon (may occur in less than 1 in 100 patients):
- Various blood cancers (after long-term treatment).
Very rare (may occur in less than 1 in 10,000 patients):
- Severe general infection (sepsis)
- Addison's disease, a condition that affects the adrenal glands, leading to brown skin discoloration, nausea, low blood pressure (feeling of fainting) and general weakness.
- Sweating, shivering and hunger due to low blood sugar levels (hypoglycemia).
- Feeling of tingling and numbness (paresthesia).
- Heart muscle weakness caused by structural changes (cardiomyopathy).
- Hives or itchy rash; skin inflammation with peeling or without (scleroderma and psoriasis), skin redness (erythema).
- Bladder inflammation, causing pain and frequent urination and a feeling of urgency to urinate with or without blood in the urine (hemorrhagic cystitis).
- General malaise (flu-like symptoms).
- Painful redness or swelling at the infection site (in case of treosulfan solution leakage into surrounding tissue). If any of the above symptoms occur, the doctor or nurse should be informed immediately.
Reporting side effects
If side effects occur, including any side effects not listed in the leaflet, tell your doctor, pharmacist, or nurse.
Side effects can be reported directly to the Department for Monitoring of Adverse Reactions to Medicinal Products of the Office for Registration of Medicinal Products, Medical Devices and Biocidal Products
Al. Jerozolimskie 181C
PL-02 222 Warsaw
Phone: + 48 22 49 21 301
Fax: + 48 22 49 21 309
Website: https://smz.ezdrowie.gov.pl
Side effects can also be reported to the marketing authorization holder. By reporting side effects, more information can be gathered on the safety of the medicine.
5. How to store Treosulfan Zentiva
The medicine should be stored out of sight and reach of children.
Do not use this medicine after the expiry date stated on the label of the vial and the carton after: Expiry date (EXP). The expiry date refers to the last day of the month.
After reconstitution, the product should not be stored in the refrigerator (2–8°C), as this may cause precipitation. Solutions with precipitate should not be used.
Do not store in the refrigerator.
The chemical and physical stability of the product has been demonstrated during use for 12 hours at 30°C. From a microbiological point of view, unless the reconstitution method excludes the risk of microbial contamination, the product should be used immediately. If the product is not used immediately, the user is responsible for the storage period and conditions during use.
Medicines should not be disposed of via wastewater or household waste.
Ask your pharmacist how to dispose of medicines that are no longer needed. This will help protect the environment.
6. Contents of the pack and other information
What Treosulfan Zentiva contains
- The active substance of the medicine is treosulfan. Each vial contains 5 g of treosulfan.
- After reconstitution, 1 ml of solution contains 50 mg of treosulfan.
What Treosulfan Zentiva looks like and contents of the pack
Treosulfan Zentiva is a white, crystalline powder or powder that is supplied in glass vials of type I; each vial contains 5 g of treosulfan.
Before administration, the dry powder is mixed with water for injections in the vial, forming a solution.
Treosulfan Zentiva is available in a carton containing 1 vial or 5 vials.
The vials may, but do not have to be, placed in a plastic sleeve with an absorbent paper backing (base). The plastic sleeve does not come into contact with the medicinal product and provides additional protection during transport. This facilitates safe handling of the medicinal product for both medical professionals and pharmaceutical personnel.
Marketing authorization holder and manufacturer
Marketing authorization holder
Zentiva, k.s.
U kabelovny 130
Dolní Měcholupy
102 37, Prague 10
Czech Republic
Importer
MIAS Pharma Limited
Suite 2, Stafford House, Strand Road
Portmarnock, Co. Dublin
Ireland
Tillomed Malta Limited,
Malta Life Sciences Park,
LS2.01.06 Industrial Estate,
San Gwann, SGN 3000, Malta
This medicinal product is authorized in the Member States of the European Economic Area under the following names:
United Kingdom:
Treosulfan 5g powder for solution for infusion
Germany:
Treosulfan Tillomed 5g Pulver zur Herstellung einer Infusionslösung
France:
Treosulfan Tillomed 5g poudre pour solution pour perfusion
Italy:
Treosulfan Tillomed
Spain:
Treosulfano Tillomed 5g polvo para solución para perfusión
EFG
Austria:
Treosulfan Tillomed 5g Pulver zur Herstellung einer Infusionslösung
Czech Republic:
Treosulfan Tillomed
Greece:
Treosulfan Tillomed 5g κόνις για διάλυμα προς έγχυση
Poland:
Treosulfan Zentiva
Romania:
Treosulfan Tillomed 5g Pulbere pentru soluţie perfuzabilă
Denmark:
Treosulfan Tillomed
Finland:
Treosulfan Tillomed infuusiokuiva-aine liuosta varten 5g
Norway:
Treosulfan Tillomed 5g Pulver til infusjonsvæske, oppløsning
Sweden:
Treosulfan Tillomed 5g Pulver till infusionsvätska, lösning
Belgium:
Treosulfan Tillomed 5g Poeder voor oplossing voor infusie
Treosulfan Tillomed 5g poudre pour solution pour perfusion
Treosulfan Tillomed 5g Pulver zur Herstellung einer Infusionslösung
Ireland:
Treosulfan Tillomed 5g powder for solution for infusion
Netherlands:
Treosulfan Tillomed 5g Poeder voor oplossing voor infusie
Portugal:
Treossulfano Tillomed 5g pó para solução para perfusão
Date of last revision of the leaflet:March 2024
------------------------------------------------------------------------------------------------------------
Information intended for healthcare professionals only:
For single use only.
Guidelines for safe handling of cytotoxic medicines:
- 1. The reconstitution of the medicinal product should be carried out by trained healthcare personnel.
- 2. This should be done in a designated area.
- 3. Protective gloves, masks, and clothing should be worn.
- 4. Precautions should be taken to avoid accidental contact of the medicinal product with the eyes. In case of accidental contact, the affected area should be rinsed with plenty of water or normal saline solution. To remove transient skin burning, a mild cream can be applied. In case of eye contact, medical advice should be sought.
- 5. Pregnant women should not handle cytotoxic preparations.
- 6. When disposing of materials (syringes, needles, etc.) used for the reconstitution of cytotoxic medicines, proper precautions and safety measures should be taken.
- 7. The work surface should be covered with a single-use plastic sheet with absorbent paper on the back.
- 8. Luer-Lock connections should be used on all syringes and sets. The use of needles with large openings is recommended to minimize pressure and the possibility of aerosol formation. This possibility can also be reduced by using a vented needle.
Instructions for reconstitution of Treosulfan Zentiva
To avoid problems with solubility during reconstitution, the following aspects should be considered:
- 1. The solvent, water for injections, should be heated to 25–30ºC (not higher) in a water bath.
- 2. Treosulfan should be carefully removed from the inner surface of the infusion vial by shaking. This procedure is very important, as wetting the powder will cause it to stick to the surface, leading to clumping. If clumping occurs, the vial should be shaken vigorously for a longer time.
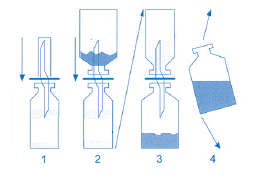
- 3. One end of the double-ended cannula should be inserted into the rubber stopper of the vial with water. The vial with treosulfan should then be inserted into the other end of the cannula, with the bottom facing up. The entire assembly should be inverted. During gentle shaking of the vial, the water flows into the vial at the bottom. If these instructions are followed, the entire reconstitution procedure should not take longer than 2 minutes. The following diagram facilitates the reconstitution process.
- Country of registration
- Active substance
- Prescription requiredYes
- ImporterMIAS Pharma Limited Tillomed Malta Limited
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to Treosulfan Zentiva
Alternatives to Treosulfan Zentiva in other countries
The best alternatives with the same active ingredient and therapeutic effect.
Alternative to Treosulfan Zentiva in Spain
Online doctors for Treosulfan Zentiva
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for Treosulfan Zentiva – subject to medical assessment and local rules.






