
TREOSULFANO ZENTIVA 5 g POWDER FOR SOLUTION FOR INFUSION

How to use TREOSULFANO ZENTIVA 5 g POWDER FOR SOLUTION FOR INFUSION
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the User
Treosulfan Zentiva 5 g powder for solution for infusion EFG
Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor or nurse.
- If you experience any side effects, talk to your doctor or nurse, even if they are not listed in this leaflet. See section 4.
Contents of the pack
- What is Treosulfan Zentiva and what is it used for
- What you need to know before you use Treosulfan Zentiva
- How to use Treosulfan Zentiva
- Possible side effects
- Storage of Treosulfan Zentiva
- Contents of the pack and other information
1. What is Treosulfan Zentiva and what is it used for
Treosulfan Zentiva contains the active substance treosulfan. Treosulfan belongs to the group of alkylating agents. These agents work by stopping the growth of cancer cells.
Treosulfan Zentiva has been prescribed to you by your doctor for the treatment of advanced ovarian cancer after at least one previous standard treatment.
2. What you need to know before you use Treosulfan Zentiva
Do not use Treosulfan Zentiva:
- if you are allergic to treosulfan;
- if you do not have enough blood cells (severe bone marrow suppression);
- if you are breast-feeding.
Before each administration, a blood test should be performed to verify that you have enough blood cells to receive Treosulfan Zentiva.
Warnings and precautions
Talk to your doctor or nurse before starting Treosulfan Zentiva:
Bone marrow damage and blood cell count monitoring
The adverse reaction that limits the dose of treosulfan is the restriction of bone marrow function, which usually disappears after treatment is discontinued. It manifests as a reduction in white blood cells (leukocytes) and platelets (thrombocytes) and a decrease in red blood cells (hemoglobin).
Since bone marrow function disorders accumulate, your doctor will monitor your blood cell count at shorter intervals from the third cycle. This is especially important when combined with other forms of therapy that affect the bone marrow, such as radiation therapy. If bone marrow function is affected, there is a higher risk of infection.
In general, white blood cells (leukocytes) and platelets (thrombocytes) return to their baseline levels after 28 days.
Pulmonary toxicity
Difficulty breathing, coughing, or high fever may indicate a lung disease. If there are severe limitations of lung function, such as inflammation, scarring, or infections, treatment with treosulfan should be discontinued.
You should consider the following aspects while being treated with Treosulfan Zentiva:
- increases your risk of developing certain types of infection;
- different types of blood cancer may occur after prolonged treatment;
- treatment with cancer medications may increase the risk of generalized infection after some vaccinations. Therefore, you should avoid being vaccinated with live vaccines;
- due to the possible development of bladder inflammation that causes pain or increased urination frequency or urgency, with or without blood in the urine (hemorrhagic cystitis), you are advised to drink more fluids than usual up to 24 hours after your treatment with treosulfan.
Extravasation
The infusion of treosulfan should be performed using a safe technique, as the extravasation of the treosulfan solution into the surrounding tissue can cause inflammatory and painful reactions at the injection site. If extravasation occurs, the infusion should be discontinued immediately and the remaining dose administered in another vein.
Other medicines and Treosulfan Zentiva
Tell your doctor if you are using, have recently used, or might use any other medicines, including those obtained without a prescription. This includes herbal medicines.
The effect of treatment with ibuprofen/chloroquine may be reduced when administered with Treosulfan Zentiva.
Pregnancy andbreast-feeding
If you are pregnant or breast-feeding, think you may be pregnant, or plan to become pregnant, consult your doctor before using this medicine. There are no data or limited data on the use of Treosulfan Zentiva 5 g powder for solution for infusion in pregnant or breast-feeding women.
Pregnancy
Because fetal damage cannot be ruled out, Treosulfan Zentiva 5 g powder for solution for infusion should not be used during pregnancy, unless your doctor considers it absolutely necessary. You should not become pregnant during treatment with Treosulfan Zentiva 5 g powder for solution for infusion.
If you become pregnant during treatment with Treosulfan Zentiva 5 g powder for solution for infusion, you should inform your doctor immediately.
Contraception in women
During and up to six months after treatment with Treosulfan Zentiva 5 g powder for solution for infusion, you should use adequate contraceptive methods if you are of childbearing age.
Breast-feeding
Since it cannot be ruled out that the substance may be transferred to breast milk, you should not breast-feed during treatment with Treosulfan Zentiva 5 g powder for solution for infusion.
Driving and using machines
No studies have been performed on the effect on the ability to drive and use machines. Do not drive and do not use machines if you have nausea and vomiting, as these effects may diminish your ability to drive or operate machinery.
3. How to use Treosulfan Zentiva
Treosulfan Zentiva is usually administered by a doctor or nurse directly into the bloodstream. Your doctor will calculate the correct dose for you and it will be administered (intravenous infusion) over 15 to 30 minutes.
Your doctor will calculate the correct dose of Treosulfan Zentiva based on your blood cell counts. Your doctor will reduce the dose if you have been given another anticancer medicine or radiation therapy. The dose will also depend on your body size and will vary according to your body surface area.
During treatment with Treosulfan Zentiva, infusions are usually administered every 3 to 4 weeks. In general, 6 cycles of treatment are given.
Your doctor may change the dose and frequency of your treatment based on the results of your blood tests, your general condition, any additional treatment you are receiving, and your response to treatment with Treosulfan Zentiva. If in doubt, ask your doctor or nurse.
If you experience pain at the injection site, inform your doctor or nurse immediately.
Use in children
It is not recommended for use in children.
If you receive more Treosulfan Zentiva than you should
If you have been given too much of this medicine, you may feel unwell or your blood cells may be reduced. Your doctor may administer a blood transfusion and take other measures if necessary.
If you have any questions about the use of this medicine, ask your doctor, pharmacist, or nurse.
In case of overdose or accidental ingestion, consult your doctor or pharmacist immediately or call the Toxicology Information Service, phone: 91 562 04 20, indicating the medicine and the amount ingested.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them. If you experience any side effect, it is important that you inform your doctor before the next treatment.
Tell your doctor immediately if you notice any of the following symptoms:
- Allergic reactions [rare (may affect up to 1 in 1000 people)]: if you develop itching, rash, swelling of the face, lips, tongue, and/or throat, which can cause difficulty swallowing or breathing or a decrease in blood pressure.
- Fever or infection [very common (may affect more than 1 in 10 people)]: if you have a body temperature of 38°C or more, experience sweating, or observe any other sign of infection (you may have fewer white blood cells than normal).
- Weakness [very common (may affect more than 1 in 10 people)], difficulty breathing, or if your skin becomes pale (you may have fewer red blood cells than normal).
- Bleeding [very common (may affect more than 1 in 10 people)]from the gums, mouth, or nose, or abnormal bruising (you may have fewer platelets than normal).
- Difficulty breathing [very rare (may affect up to 1 in 10,000 people)](you may have an allergic reaction, inflammation, or lung infection).
Very common side effects (may affect more than 1 in 10 people):
- Reduction of white blood cells (increases the risk of infection), platelets (may cause bruising and bleeding from the gums and mouth and nose), and red blood cells (may cause paleness, weakness, and difficulty breathing) - therefore, blood cell counts should be regularly monitored.
- Stomach problems, including nausea (feeling sick) with or without vomiting (being sick).
- Mild hair loss. After your treatment, normal hair growth returns.
- Bronze discoloration of the skin.
Common side effects (may affect up to 1 in 10 people):
- Infections caused by fungi, viruses, or bacteria (may lead to fever, sweating, and a general feeling of discomfort).
- Gastrointestinal disorders.
Uncommon side effects (may affect up to 1 in 100 people):
- Different types of blood cancer (after prolonged treatments).
- Inflammation of the oral mucous membrane (stomatitis).
Rare side effects (may affect up to 1 in 10,000 people):
- Severe and simultaneous reduction of all blood cells (pancytopenia); may cause weakness and bruising and increase the risk of infection.
- Addison's disease, a disease in which the adrenal glands do not function properly, resulting in bronzed skin, stomach upset, low blood pressure (dizziness), and a general feeling of weakness.
- Sweating, trembling, and hunger due to a decrease in blood glucose levels (hypoglycemia).
- Numbness and tingling (paresthesia).
- Cardiac muscle weakness caused by a structural change (cardiomyopathy).
- Difficulty breathing (inflammation and scarring of the lungs and lung infections).
- Increased liver function values (may cause fatigue, feeling of pressure in the upper right abdomen, and yellowing of the sclera and skin).
- Hives or rash with itching; skin inflammation with or without scaling (scleroderma and psoriasis), redness of the skin (erythema).
- Bladder inflammation, which causes pain or increased urination frequency or urgency, with or without blood in the urine (hemorrhagic cystitis).
- Feeling of discomfort (symptoms similar to those of the flu).
- Painful redness or swelling at the injection site (in case the treosulfan solution comes into contact with the skin).
Frequency not known: cannot be estimated from the available data
- Septicemia (sepsis).
Tell your doctor or nurse immediately if you notice any of the above symptoms.
Reporting of side effects
If you experience any side effects, talk to your doctor or nurse, even if they are not listed in this leaflet. You can also report them directly through the Spanish Pharmacovigilance System for Human Use Medicines: https://www.notificaram.es. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of Treosulfan Zentiva
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date which is stated on the carton after EXP. The expiry date refers to the last day of the month shown.
Once reconstituted, do not store the medicine in the refrigerator (2 - 8°C) because it may precipitate. Do not use if the solution contains precipitates.
Do not refrigerate.
The physical and chemical stability in use has been demonstrated for 12 hours at 30°C. From a microbiological point of view, unless the dilution has been carried out under controlled and validated aseptic conditions, the product should be used immediately. If not, the in-use storage times and conditions are the responsibility of the user.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. This will help protect the environment.
6. Container Content and Additional Information
Composition of Treosulfan Zentiva
- The active ingredient is treosulfan. Each vial contains 5 g of treosulfan.
- After reconstitution, each milliliter of solution contains 50 mg of treosulfan
Appearance of the Product and Container Content
White crystalline powder or compact aggregate that is supplied in transparent glass vials, each vial contains 5 g of treosulfan.
The powder is reconstituted with water for injectable preparations in the vial forming a solution before administration.
Treosulfan Zentiva is available in packs with 1 vial or 5 vials.
Vials with or without a retractable plastic wrapper with a protective base (disk). The retractable wrapper is not in contact with the product and provides additional protection during transport. It also improves the safe handling of the product by healthcare professionals.
Marketing Authorization Holder
Zentiva k.s.,
U kabelovny 130,
Prague 10 – Dolní Mecholupy,
102 37 Czech Republic
Manufacturer
MIAS Pharma Limited
Suite 2, Stafford House, Strand Road
Portmarnock, Co. Dublin
Ireland
Tillomed Malta Limited,
Malta Life Sciences Park,
LS2.01.06 Industrial Estate,
San Gwann, SGN 3000, Malta
Further information about this medicinal product can be obtained from the local representative of the marketing authorization holder:
Zentiva Spain S.L.U.
Avenida de Europa, 19, Edificio 3, Planta 1.
28224 Pozuelo de Alarcón, Madrid
Spain
This medicinal product is authorized in the Member States of the European Economic Area under the following names:
Germany | Treosulfan Tillomed 5g Powder for Solution for Infusion |
France | Treosulfan Tillomed 5g Powder for Solution for Perfusion |
Spain | Treosulfano Zentiva 5 g Powder for Solution for Infusion EFG |
Austria: | Treosulfan Tillomed 5g Powder for Solution for Infusion |
Czech Republic: | Treosulfan Tillomed |
Greece: | Treosulfan Tillomed 5g κ?νις για δι?λυμα προς ?γχυση |
Poland: | Treosulfan Tillomed |
Romania: | Treosulfan Tillomed 5g Pulbere pentru solutie perfuzabila |
Denmark: | Treosulfan Tillomed |
Finland: | Treosulfan Tillomed infuusiokuiva-aine liuosta varten 5g |
Norway: | Treosulfan Tillomed 5g Pulver til infusjonsvæske, oppløsning |
Sweden: | Treosulfan Tillomed 5g Pulver till infusionsvätska, lösning |
Belgium: | Treosulfan Tillomed 5g Poeder voor oplossing voor infusie Treosulfan Tillomed 5g poudre pour solution pour perfusion Treosulfan Tillomed 5g Pulver zur Herstellung einer Infusionslösung |
Ireland: | Treosulfan Tillomed 5g powder for solution for infusion |
Netherlands: | Treosulfan Tillomed 5g Poeder voor oplossing voor infusie |
Portugal: | Treossulfano Tillomed 5g pó para solução para perfusão |
Date of the Last Revision of thisLeaflet:May 2025
Detailed information on this medicinal product is available on the website of the Spanish Agency for Medicines and Health Products (AEMPS) (http://www.aemps.gob.es/)
--------------------------------------------------------------------------------------------------------
This information is intended only for healthcare professionals:
For single use.
Procedures for Handling and Disposal of Cytotoxic Medicines:
- Only trained personnel should reconstitute the solution.
- A dedicated preparation area is required for this purpose.
- Personnel must wear suitable protective clothing, masks, and gloves.
- Precautions must be taken to avoid accidental contact of the medicinal product with the eyes. If the solution comes into contact with the skin or eyes, rinse the area immediately with plenty of water or saline solution. A cream can be used to treat transient skin irritation. In case of eye irritation, contact an ophthalmologist.
- Pregnant women should not handle this medicinal product.
- Adequate precautions must be taken for the disposal of items used to reconstitute cytotoxic medicines (syringes, needles, etc.).
- The work surface should be covered with disposable plastic and absorbent paper.
- Luer-lock fittings should be used for all equipment and syringes. Large-caliber needles are recommended to minimize pressure and possible aerosol formation. The latter can also be reduced by using a vented needle.
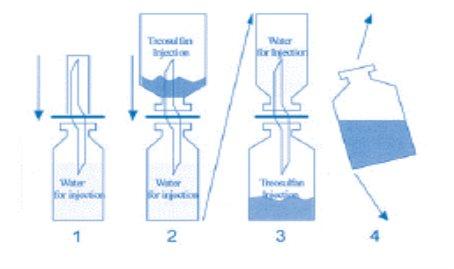
Instructions for the Reconstitution of Treosulfan Zentiva
To avoid solubility problems during reconstitution, the following aspects should be considered:
- The solvent, water for injectable preparations, should be heated to 25 - 30° C using a water bath.
- The treosulfan should be carefully removed from the inner surface of the infusion vial by shaking.
This procedure is very important, as moistening the powder that adheres to the surface results in clumping. If clumping occurs, the vial should be shaken vigorously.
- One end of the cannula is placed in the rubber stopper of the water vial and the other end of the cannula is placed in the treosulfan vial upside down.
Turn the entire system to allow the water to enter the lower vial while gently shaking.
Following these instructions, the reconstitution procedure should not take more than 2 minutes. See the diagram below to help with the reconstitution process:
- Country of registration
- Availability in pharmacies
Supply issue reported
Data from the Spanish Agency of Medicines (AEMPS) indicates a supply issue affecting this medicine.<br><br>Availability may be limited in some pharmacies.<br><br>For updates or alternatives, consult your pharmacist. - Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to TREOSULFANO ZENTIVA 5 g POWDER FOR SOLUTION FOR INFUSIONDosage form: INJECTABLE PERFUSION, 5 gActive substance: treosulfanManufacturer: Medac Gesellschaft Für Klinische Spezialpräparate GmbhPrescription requiredDosage form: INJECTABLE PERFUSION, 6 mg/mlActive substance: busulfanManufacturer: Accord Healthcare S.L.U.Prescription requiredDosage form: TABLET, 2 mg busulfanActive substance: busulfanManufacturer: Aspen Pharma Trading LimitedPrescription required
Online doctors for TREOSULFANO ZENTIVA 5 g POWDER FOR SOLUTION FOR INFUSION
Discuss questions about TREOSULFANO ZENTIVA 5 g POWDER FOR SOLUTION FOR INFUSION, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions






