
Timoptic 0,5%

Ask a doctor about a prescription for Timoptic 0,5%

How to use Timoptic 0,5%
Package Leaflet: Information for the Patient
TIMOPTIC 0.5%, 5 mg/ml, eye drops, solution
Timolol
Read the package leaflet carefully before using the medicine, as it contains important information for the patient.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their symptoms are the same as yours.
- If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the Package Leaflet
- 1. What Timoptic is and what it is used for
- 2. Important information before using Timoptic
- 3. How to use Timoptic
- 4. Possible side effects
- 5. How to store Timoptic
- 6. Contents of the pack and other information
1. What Timoptic is and what it is used for
Timoptic is a medicine that contains a beta-adrenergic receptor inhibitor that lowers the pressure inside the eyeball.
Timoptic is used to lower high pressure in the eye in patients with ocular hypertension and chronic open-angle glaucoma.
2. Important information before using Timoptic
When not to use Timoptic:
- if you are allergic to the active substance, other beta-adrenergic receptor inhibitors, or any of the other ingredients of this medicine (listed in section 6);
- in patients with certain severe breathing problems, such as asthma, or a history of asthma;
- in patients with chronic obstructive pulmonary disease (severe lung disease that can cause wheezing, difficulty breathing, and/or a persistent cough);
- in patients with a slow heart rate (bradycardia), overt heart failure, irregular heart rhythms, or cardiogenic shock.
If you have any of these conditions, you should consult your doctor before taking the medicine.
Warnings and precautions
Before starting treatment with Timoptic, you should discuss it with your doctor or pharmacist.
Tell your doctor if you have any medical conditions, including:
- heart problems (such as coronary heart disease, heart failure, low blood pressure);
- abnormal heart rhythms;
- Raynaud's disease or Raynaud's syndrome;
- lung problems or breathing difficulties (such as asthma or chronic obstructive pulmonary disease);
- diabetes or other blood sugar problems (as timolol may mask the symptoms of low blood sugar);
- hyperthyroidism (as timolol may mask the symptoms of hyperthyroidism).
Tell your doctor if you are going to have surgery, as Timoptic may affect the way some medicines used during anesthesia work.
If you are allergic to any medicines, tell your doctor.
If you suspect an allergic reaction (such as rash, redness, and itching of the eyes), stop using the medicine and see a doctor immediately.
While using Timoptic, tell your doctor if you get an eye infection, injury, or plan to have eye surgery, or if you get new symptoms or if your symptoms get worse.
Like other eye medicines, Timoptic may be absorbed into the bloodstream.
Side effects seen with beta-adrenergic receptor inhibitors given orally may also occur when used in the eye.
Patients who are already taking oral beta-adrenergic receptor inhibitors and Timoptic should be monitored for possible additive effects on intraocular pressure or known systemic effects of beta-adrenergic receptor inhibitors. Concomitant use of two topical beta-adrenergic receptor inhibitors is not recommended.
There have been reports of corneal edema, corneal haze, and iritis following the use of eye drops containing beta-adrenergic receptor inhibitors, including timolol, after ocular surgery.
Children and adolescents
Children and adolescents should use products with a lower concentration of timolol than Timoptic 0.5%.
Timoptic 0.5%
Timolol eye drops should be used with caution in children and adolescents.
In newborns, infants, and small children, timolol should be used with special caution. If coughing, wheezing, abnormal breathing, or pauses in breathing (apnea) occur, the medicine should be stopped immediately.
A doctor should be consulted as soon as possible. A portable apnea monitor may be useful.
Timolol has been studied in infants and children aged 12 days to 5 years with elevated intraocular pressure or diagnosed with glaucoma. For more information, consult your doctor.
Timoptic and other medicines
Tell your doctor about all medicines you are taking, including eye drops, and any medicines you plan to take, including those available without a prescription. It is especially important to tell your doctor if you are taking medicines to lower blood pressure or to treat heart problems, diabetes, or depression (such as fluoxetine and paroxetine).
When taken with certain other medicines (such as quinidine, selective serotonin reuptake inhibitors), timolol has been associated with increased reactions, such as slowed heart rate and depression.
Concomitant use of Timoptic with orally administered calcium channel blockers, catecholamine-depleting medicines, antiarrhythmic medicines, parasympathomimetics, or beta-adrenergic receptor inhibitors may result in decreased blood pressure and/or marked slowing of heart rate.
Oral beta-adrenergic receptor inhibitors may enhance the hypotensive effect of clonidine and may mask the signs of hypoglycemia.
Pregnancy and breastfeeding
If you are pregnant or breastfeeding, think you may be pregnant, or are planning to have a baby, ask your doctor or pharmacist for advice before taking this medicine.
Tell your doctor if you are pregnant or planning to become pregnant. Do not use Timoptic during pregnancy unless your doctor decides it is necessary.
Do not use Timoptic while breastfeeding. Timolol may pass into breast milk. Tell your doctor if you are breastfeeding.
Driving and using machines
There are side effects associated with the use of Timoptic that may affect your ability to drive or use machines (see section 4). Do not drive or use machines until the symptoms have completely resolved.
Timoptic contains benzalkonium chloride and phosphates
This medicine contains approximately 0.003 mg of benzalkonium chloride per 1 drop, equivalent to 0.10 mg/ml.
Benzalkonium chloride may be absorbed by soft contact lenses and may change their color. Remove contact lenses before administering the drops and wait at least 15 minutes before putting them back in.
Benzalkonium chloride may also cause eye irritation, especially in people with dry eye syndrome or disorders affecting the cornea (the transparent layer on the front of the eye). If you experience any abnormal sensations in the eye, stinging, or pain in the eye after using the medicine, consult your doctor.
This medicine contains approximately 0.3 mg of phosphates per 1 drop, equivalent to 11.8 mg/ml.
In patients with severe damage to the transparent, front part of the eye (cornea), phosphates may rarely cause corneal clouding due to calcium deposits during treatment.
3. How to use Timoptic
Use this medicine exactly as your doctor has told you.
If you are not sure, ask your doctor or pharmacist.
Children and adolescents
Children and adolescents should use products with a lower concentration of timolol than Timoptic 0.5%.
Timoptic 0.5%
Before using timolol, the doctor should perform a thorough medical examination.
The doctor will carefully assess the risk-benefit ratio of using timolol. If the benefits outweigh the risks, the medicine should be administered at the lowest available concentration of timolol (0.1%) once a day.
In children, a 0.1% solution is usually sufficient to lower intraocular pressure. If this dose does not sufficiently lower intraocular pressure, it may be necessary to administer the medicine twice a day, with a 12-hour interval. Children, especially newborns, should be closely monitored for 1 to 2 hours after the first dose and carefully monitored for side effects until surgery is performed.
Treatment period
In children and adolescents, timolol is used for transitional treatment.
Adults
In initial treatment in adults, products with a lower concentration of timolol than Timoptic 0.5% are used.
The usual initial dose is one drop of 0.25% timolol solution in the affected eye(s) in the morning and evening. In some cases, the doctor may increase the dose to one drop of Timoptic 0.5% in the affected eye(s) in the morning and evening.
If the patient's intraocular pressure is stabilized, the doctor may recommend using Timoptic once a day.
Do not change the dose prescribed by your doctor. If you need to stop using the medicine, consult your doctor immediately.
In some cases, the doctor may recommend using other medicines, including other eye drops, to lower intraocular pressure.
If you are using another eye medicine, wait at least 15 minutes between administering the different medicines. Eye ointments should always be administered last.
Do not touch the tip of the dropper bottle to the eye or its surroundings. This may contaminate the solution with bacteria and lead to eye infection, resulting in serious damage or even vision loss. To avoid possible contamination of the solution, avoid contact between the dropper and any surface.
Instructions for use
Do not use if the plastic safety strip around the neck of the bottle is damaged or missing. When opening the bottle for the first time, remove the plastic safety strip.
Every time you use Timoptic:
- 1. Wash your hands.
- 2. Open the bottle. Be careful not to touch the dropper to the eye, the skin around the eye, or your fingers.Failing to follow these instructions may lead to contamination of the eye drops. Using contaminated eye drops can lead to serious complications and even vision loss.
- 3. Tilt your head back and hold the bottle upside down over the eye.
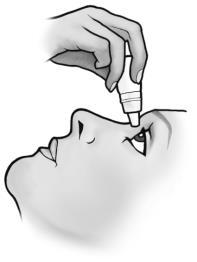
- 4. Pull the lower eyelid down and look up. Hold the bottle and gently squeeze the sides to release a single drop into the space between the lower eyelid and the eye.
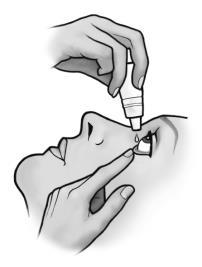
- 5. After administering the medicine, release the lower eyelid. Press the finger into the corner of the eye, by the nose, and gently close the eye for 3-5 minutes. This helps prevent the medicine from entering the entire body.
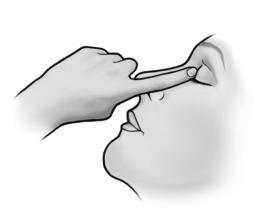
- 6. To administer the medicine to the other eye, if instructed by your doctor, repeat steps 3 to 5.
- 7. Replace the cap and tighten the bottle.
Using more Timoptic than prescribed
If you accidentally administer too much medicine or swallow the contents of the bottle, you may experience dizziness, difficulty breathing, or a feeling of slowed heart rate.
Consult your doctor immediately.
Missing a dose of Timoptic
Use Timoptic as prescribed by your doctor. If you miss a dose, use it as soon as possible. However, if it is almost time for your next dose, do not use the missed dose, and continue with your regular dosing schedule.
Do not use a double dose to make up for a missed dose.
4. Possible side effects
Like all medicines, Timoptic can cause side effects, although not everybody gets them.
If you experience an allergic reaction, including hives, face, lip, tongue, and/or throat swelling, which may cause difficulty breathing or swallowing, stop using the medicine and contact your doctor or the Emergency Department of your nearest hospital immediately.
As with other eye medicines, timolol is absorbed into the bloodstream. It may cause the same types of side effects as beta-adrenergic receptor inhibitors given orally or intravenously. The frequency of systemic side effects after topical administration to the eye is lower than after oral administration or injection.
The following side effects include those seen with the entire group of beta-adrenergic receptor inhibitors used in the eye.
During clinical trials and after the medicine was marketed, the following side effects were observed after topical administration of timolol maleate to the eye in any form.
Common (affecting 1 to 10 in 100 patients):
- headache
- eye irritation symptoms, including burning and stinging, itching, tearing, redness, conjunctivitis, blepharitis, keratitis, decreased corneal sensitivity, and dry eye
Uncommon (affecting 1 to 10 in 1,000 patients):
- dizziness, depression
- visual disturbances, including refractive errors (in some cases due to the withdrawal of miotic therapy)
- slow heart rate, fainting
- shortness of breath
- nausea, gastrointestinal disturbances
- weakness, feeling tired
Rare (affecting 1 to 10 in 10,000 patients):
- allergic reaction symptoms, including anaphylaxis, angioedema, hives, localized or generalized rash
- insomnia, nightmares, memory loss, feeling of pins and needles, exacerbation of myasthenia gravis, stroke, decreased libido
- double vision, drooping eyelid, detachment of the retina after filtration surgery
- tinnitus
- arrhythmia, decreased blood pressure, chest pain, heart block, cerebral ischemia, congestive heart failure, tachycardia, cardiac arrest, edema, Raynaud's phenomenon
- bronchospasm (especially in patients with pre-existing bronchospastic disease), respiratory failure, cough
- diarrhea, dry mouth
- alopecia, psoriasiform rash or exacerbation of psoriasis
- systemic lupus erythematosus
- Peyronie's disease (hardening of the penis, which can cause curved, painful erections)
Frequency not known (cannot be estimated from the available data):
- abdominal pain, vomiting, taste disturbances
- muscle pain
- sexual dysfunction, decreased libido
- hallucinations
- hypoglycemia
- pruritus, skin rash
- syncope, stroke, cerebral ischemia
- blurred vision
Clinical laboratory test results:clinically significant changes in routine laboratory tests were rarely associated with oral timolol maleate. Minor increases in blood urea nitrogen, serum potassium, uric acid, and triglycerides, as well as minor decreases in hemoglobin, hematocrit, and HDL-cholesterol, occurred but were not progressive or associated with clinical symptoms.
Side effects seen with oral timolol maleate may also occur with topical timolol maleate.
Reporting side effects
If you experience any side effects, including those not listed in this leaflet, tell your doctor or pharmacist. You can also report side effects directly to the Department of Drug Safety Monitoring, Office for Registration of Medicinal Products, Medical Devices, and Biocidal Products, Al. Jerozolimskie 181C, 02-222 Warsaw, phone: +48 22 49 21 301, fax: +48 22 49 21 309, website: https://smz.ezdrowie.gov.pl.
Side effects can also be reported to the marketing authorization holder.
By reporting side effects, you can help provide more information on the safety of this medicine.
5. How to store Timoptic
Keep this medicine out of the sight and reach of children.
Store in a temperature below 30°C.
Store in the original package to protect from light.
Do not use this medicine after the expiry date stated on the packaging after "EXP".
The expiry date refers to the last day of the month stated.
Shelf life after opening the bottle: 4 weeks.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. This will help protect the environment.
6. Contents of the pack and other information
What Timoptic contains
- The active substance is timolol (as timolol maleate), 5 mg/ml.
- The other ingredients are: sodium dihydrogen phosphate dihydrate (as monohydrate), disodium phosphate dodecahydrate (as anhydrous), benzalkonium chloride 50% solution (as benzalkonium chloride), sodium hydroxide 1N solution, water for injections.
See section 2 "Timoptic contains benzalkonium chloride and phosphates".
What Timoptic looks like and contents of the pack
Timoptic is available in bottles containing 5 ml of sterile eye drop solution, in a cardboard box.
Marketing authorization holder and manufacturer
Marketing authorization holder
Santen Oy
Niittyhaankatu 20
33720 Tampere
Finland
Manufacturer
Laboratoires Merck Sharp & Dohme – Chibret
Mirabel Plant, Route de Marsat – Riom
63963 Clermont-Ferrand
Cedex 9, France
Santen Oy
Kelloportinkatu 1
33100 Tampere
Finland
Date of last revision of the leaflet:
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- ImporterLaboratoires Merck Sharp & Dohme - Chibret Mirabel Plant Santen OY
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to Timoptic 0,5%Dosage form: Drops, 5 mg/mlActive substance: timololManufacturer: SIEGFRIED El Masnou, S.A.Prescription requiredDosage form: Drops, 2.5 mg/mlActive substance: timololManufacturer: Warszawskie Zakłady Farmaceutyczne POLFA S.A.Prescription requiredDosage form: Drops, 5 mg/mlActive substance: timololManufacturer: Warszawskie Zakłady Farmaceutyczne POLFA S.A.Prescription required
Alternatives to Timoptic 0,5% in other countries
The best alternatives with the same active ingredient and therapeutic effect.
Alternative to Timoptic 0,5% in Spain
Alternative to Timoptic 0,5% in Ukraine
Online doctors for Timoptic 0,5%
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for Timoptic 0,5% – subject to medical assessment and local rules.














