
GELISIA 1 mg/g OPHTHALMIC GEL

How to use GELISIA 1 mg/g OPHTHALMIC GEL
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the User
Gelisia 1mg/g eye gel in single-dose container
timolol
Read the entire package leaflet carefully before starting to use this medication, as it contains important information for you.
- Keep this package leaflet, as you may need to read it again.
- If you have any questions, consult your doctor, pharmacist, or nurse.
- This medication has been prescribed to you only, and you should not give it to others, even if they have the same symptoms as you, as it may harm them.
- If you experience side effects, consult your doctor, pharmacist, or nurse, even if they are not listed in this package leaflet. See section 4.
Contents of the Package Leaflet
- What is Gelisia and what is it used for
- What you need to know before starting to use Gelisia
- How to use Gelisia
- Possible side effects
5 Conservation of Gelisia
- Container contents and additional information
1. What is Gelisia and what is it used for
Gelisia is a beta-blocker that is administered in the eye.
This medication is used to treat certain types of eye diseases that involve high pressure in the eye (glaucoma and ocular hypertension).
2. What you need to know before starting to use Gelisia
Do not use Gelisia
- if you are allergic to timolol maleate, beta-blockers, or any of the other components of this medication (listed in section 6);
- if you currently have or have had respiratory problems such as asthma, severe chronic obstructive bronchitis (severe lung disease that can cause wheezing, difficulty breathing, and/or prolonged coughing);
- if you have a slow heart rate, heart failure, or heart rhythm disorders (irregular heartbeats);
- in the case of untreated pheochromocytoma (high blood pressure caused by high levels of a hormone);
- in the case of corneal dystrophies (degenerative disorders of the cornea).
Warnings and Precautions
- Consult your doctor or pharmacist before starting to use Gelisia.
Do not stop treatment abruptly without your doctor's advice.
Do not inject or swallow it.
If you use this medication, you should undergo regular checks of intraocular pressure and cornea.
Before using this medication, inform your doctor if you currently have or have had:
- coronary artery disease (symptoms may include chest pain or tightness, shortness of breath, or choking), heart failure, low blood pressure;
- heart rhythm disorders such as slow heartbeats;
- respiratory problems, asthma, or chronic obstructive pulmonary disease (lung disease that can cause wheezing, difficulty breathing, and/or prolonged coughing);
- diseases that cause poor blood circulation (such as Raynaud's disease or Raynaud's syndrome);
- diabetes, as timolol maleate may mask the signs and symptoms of low blood sugar;
- hyperthyroidism, as timolol maleate may mask the signs and symptoms;
- treated pheochromocytoma;
- psoriasis;
- corneal disease;
- metabolic disease.
Precautions for contact lens use
Avoid using contact lenses during treatment because fewer tears will be secreted; this is usually related to beta-blockers.
If you need to undergo any type of surgery, inform your doctor that you are using this medication, as timolol maleate may alter the effects of some medications used during anesthesia.
Other Medications and Gelisia
Inform your doctor or pharmacist if you are using, have recently used, or may need to use any other medication.
If your doctor has prescribed another type of eye drop, you should apply this eye drop 15 minutes before Gelisia.
This medication may affect or be affected by other medications you are using, including other eye drops for the treatment of glaucoma.
Inform your doctor if you are using or plan to use medications to lower blood pressure, heart medications, or medications to treat diabetes, quinidine (used to treat heart diseases and some types of malaria), or antidepressants known as fluoxetine and paroxetine.
Pregnancy and Breastfeeding
If you are pregnant or breastfeeding, think you may be pregnant, or plan to become pregnant, consult your doctor or pharmacist before using this medication.
Do not use this medication if you are pregnant, unless your doctor considers it necessary.
Do not use this medication if you are breastfeeding. Timolol maleate may pass into breast milk.
Consult your doctor before taking any medication during breastfeeding.
Driving and Using Machines
You may experience temporary blurred vision after using the medication. Wait until your vision returns to normal before driving or using any machine.
Gelisia may cause other side effects (dizziness, fatigue) that may affect your ability to drive or use machines. If in doubt, consult your doctor.
Sports
Administration of the medication may result in a positive doping test result.
3. How to Use Gelisia
Follow the administration instructions for this medication exactly as indicated by your doctor or pharmacist. If in doubt, consult your doctor or pharmacist again.
The recommended dose is one drop in the affected eye(s) once a day in the morning. Each single-dose container contains enough gel to treat both eyes.
Use in Children and Adolescents
There is no experience in children and adolescents. Therefore, the use of this eye gel is not recommended in these patients.
Method of Administration
This medication is intended for administration in the eye (ophthalmic route).
For single use only.
- Wash/disinfect your hands.
- Open the aluminum pouch containing the single-dose containers.
- Separate a single-dose container from the strip and put the unopened containers back in the aluminum pouch.

- Make sure the single-dose container is intact before using it.
- Hold the single-dose container upside down and gently shake it back and forth.
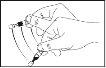
- Open by twisting the top and pulling it off. Do not touch the tip after opening the container.

- Gently squeeze until one drop falls into the space between the eyeball and the lower eyelid. To avoid contamination, never touch the dropper tip of the container to any surface.

After using the Gelisia eye gel, press with a finger on the inner corner of the eye, next to the nose, for 2 minutes. This helps prevent timolol maleate from passing into the rest of the body.
- Discard the single-dose container after use.
If you use more Gelisia than you should
Among other effects, you may feel dizzy, or you may have difficulty breathing or feel that your pulse has slowed down.
Consult your doctor or pharmacist immediately.
In case of overdose or accidental ingestion, consult your doctor or pharmacist immediately or call the Toxicology Information Service, phone 91 5620420, indicating the medication and the amount ingested.
If you forget to use Gelisia
Do not use a double dose to make up for forgotten doses. Apply the forgotten dose as soon as possible. However, if it is close to the time of the next dose, simply omit the forgotten dose.
If you stop treatment with Gelisia
The pressure inside the eye may increase and damage vision.
Do not stop treatment abruptly without your doctor's advice.
If you have any other questions about the use of this medication, ask your doctor or pharmacist.
4. Possible Side Effects
Like all medications, this medication can cause side effects, although not everyone will experience them.
Usually, you can continue using the drops, unless the effects are severe. If you are concerned, consult your doctor or pharmacist. Do not stop using this medication without talking to your doctor first.
Like other medications that are administered in the eyes, timolol maleate is absorbed into the bloodstream. This can cause side effects similar to those observed with intravenous and/or oral beta-blockers. The incidence of side effects after topical ocular administration is lower than when medications are taken, for example, orally or injected.
The listed side effects include reactions observed within the class of beta-blockers when used to treat eye conditions. The frequency of individual side effects listed cannot be estimated from the available data:
- Systemic lupus erythematosus, generalized allergic reactions that include swelling under the skin (which can occur in areas such as the face and limbs, and can obstruct the airways, causing difficulty swallowing or breathing), urticaria (or itchy rash), localized and generalized rash, itching, severe and sudden allergic reaction that is life-threatening.
- Low blood sugar levels.
- Difficulty sleeping (insomnia), depression, nightmares, memory loss, hallucinations.
- Fainting, stroke, reduced blood flow to the brain, worsening of myasthenia gravis symptoms (muscle disorder), dizziness, unusual sensations (such as tingling) and headache.
- Signs and symptoms of eye irritation (e.g., burning, stinging, itching, tearing, redness), conjunctival redness, conjunctivitis, eyelid inflammation, corneal inflammation, blurred vision, and detachment of the layer under the retina that contains blood vessels after filtration surgery, which can cause visual disturbances, decreased corneal sensitivity, dry eyes, corneal erosion (damage to the outer layer of the eyeball), drooping of the upper eyelid (which makes the eye stay half-closed), double vision, changes in the way the eye sees where objects are (refraction, sometimes due to interruption of treatment with miotic drops).
- Slow heart rate, chest pain, palpitations, edema (fluid accumulation), changes in heart rate or rhythm, congestive heart failure (heart disease with difficulty breathing and swelling of the feet and legs due to fluid accumulation), a type of heart rhythm disorder, myocardial infarction, heart failure, cramps in the legs and/or pain in the legs when walking (claudication).
- Low blood pressure, Raynaud's phenomenon, cold hands and feet.
- Narrowing of the pulmonary airways (predominantly in patients with pre-existing diseases), difficulty breathing, coughing.
- Taste disturbances, nausea, indigestion, diarrhea, dry mouth, abdominal pain, vomiting.
- Hair loss, skin rash with a white plate-like appearance (psoriasiform rash) or worsening of psoriasis, skin rash.
- Muscle pain not caused by exercise.
- Sexual dysfunction, decreased libido, impotence.
- Muscle weakness/fatigue.
- Positive results for antinuclear antibodies.
Reporting Side Effects
If you experience any side effects, consult your doctor, pharmacist, or nurse, even if they are not listed in this package leaflet. You can also report them directly through the Spanish Medication Pharmacovigilance System for Human Use: https://www.notificaram.es. By reporting side effects, you can contribute to providing more information on the safety of this medication.
5. Conservation of Gelisia
Keep this medication out of the sight and reach of children.
Do not use this medication after the expiration date that appears on the box, on the pouch, and on the single-dose container after CAD. The expiration date is the last day of the month indicated.
Do not store above 25°C.
Keep the single-dose containers in the pouch and in the outer packaging to protect them from light.
The medication does not contain preservatives. Once opened, Gelisia should be used immediately; any remaining amount should be discarded.
Once the pouch is opened: use the single-dose containers before 1 month.
Medications should not be thrown away through the wastewater or the trash. Deposit the containers and medications you no longer need in the SIGRE collection point at the pharmacy. If in doubt, ask your pharmacist how to dispose of the containers and medications you no longer need. This way, you will help protect the environment.
6. Container Contents and Additional Information
Composition of Gelisia
- The active ingredient is timolol. Each gram of gel contains 1 mg of timolol as timolol maleate.
- The other components are sorbitol (E 420), poly(vinyl alcohol), carbomer 974P, sodium acetate trihydrate, L-lysine monohydrate, and purified water.
Appearance of the Product and Container Contents
Gelisia is an opalescent gel that is colorless to slightly yellow, containing 5 single-dose containers that are inserted into a polyester/aluminum/polyethylene pouch and packaged in a box. Each single-dose container contains 0.4 g of eye gel.
Each box contains 10, 30, or 90 single-dose containers.
Only some package sizes may be marketed.
Marketing Authorization Holder and Manufacturer
SIFI S.p.A.
Via Ercole Patti 36
95025 Aci Sant'Antonio (CT)
Italy
Date of the Last Revision of this Package Leaflet:03/2023.
You can access detailed information about this medication by scanning the QR code included in the package leaflet and on the box with your smartphone. You can also access this information on the following website: Spanish Agency for Medicines and Health Products (AEMPS) (http://www.aemps.gob.es/).
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to GELISIA 1 mg/g OPHTHALMIC GELDosage form: EYE DROP, 5 mg/mlActive substance: timololManufacturer: Immedica Pharma AbPrescription requiredDosage form: EYE DROP, 0.25%Active substance: timololManufacturer: Laboratorios Thea S.A.Prescription required
Online doctors for GELISIA 1 mg/g OPHTHALMIC GEL
Discuss questions about GELISIA 1 mg/g OPHTHALMIC GEL, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions















