
Cusimolol 0,5%

Ask a doctor about a prescription for Cusimolol 0,5%

How to use Cusimolol 0,5%
Leaflet accompanying the packaging: patient information
Cusimolol 0.5%, 5 mg/ml, eye drops, solution
Timolol
Read the leaflet carefully before using the medicine, as it contains important information for the patient.
- Keep this leaflet, so you can read it again if you need to.
- If you have any doubts, consult your doctor or pharmacist.
- This medicine has been prescribed specifically for you. Do not pass it on to others. The medicine may harm another person, even if their symptoms are the same. If the patient experiences any side effects, including any possible side effects not listed in this leaflet, they should tell their doctor or pharmacist. See section 4.
Table of contents of the leaflet
- 1. What is Cusimolol 0.5% and what is it used for
- 2. Important information before using Cusimolol 0.5%
- 3. How to use Cusimolol 0.5%
- 4. Possible side effects
- 5. How to store Cusimolol 0.5%
- 6. Contents of the packaging and other information
1. What is Cusimolol 0.5% and what is it used for
Cusimolol 0.5% eye drops are a solution. Cusimolol 0.5% contains the active substance maleic acid timolol, which belongs to the group of beta-adrenergic blocking agents. The action of the medicine is to reduce the pressure inside the eye (intraocular pressure). Cusimolol 0.5% is indicated for the treatment of increased intraocular pressure in conditions such as glaucoma, chronic open-angle glaucoma, including glaucoma in patients with aphakic eyes. The doctor will explain the nature of the disease to the patient and justify the need to use Cusimolol 0.5%.
2. Important information before using Cusimolol 0.5%
When not to use Cusimolol 0.5%
If the patient finds that any of the above contraindications apply to them, they should inform their doctor before using the medicine and then follow the doctor's instructions.
Warnings and precautions
Before starting treatment with Cusimolol 0.5%, the patient should discuss it with their doctor, pharmacist, or nurse if they currently have or have had:
- ischemic heart disease (symptoms may include chest pain or tightness, shortness of breath, or choking), heart failure, low blood pressure;
- heart rhythm disorders, such as slow heart rate;
- breathing problems, asthma, or chronic obstructive pulmonary disease;
- diseases related to poor blood circulation (such as Raynaud's disease or Raynaud's syndrome);
- diabetes, as timolol may mask the symptoms of low blood sugar;
- hyperthyroidism, as timolol may mask the symptoms of hyperthyroidism.
Cusimolol 0.5% may be absorbed systemically and may cause side effects, such as those that occur with oral beta-adrenergic blocking agents. If the patient has myasthenia (chronic muscle-nerve weakness), they should consult their doctor before starting treatment with Cusimolol 0.5% and follow their instructions. Cusimolol 0.5% may exacerbate symptoms. In the event of an acute allergic reaction (skin rash, redness, eye itching) while using Cusimolol 0.5%, regardless of the cause of the allergic reaction, standard treatment with epinephrine (adrenaline) may be ineffective. Therefore, if the patient is using other medicines, they should tell their doctor about using Cusimolol 0.5%. If an eye infection or injury occurs while using Cusimolol 0.5%, the patient should inform their doctor:
- it is not recommended to administer two topical beta-adrenergic blocking agents simultaneously. If the patient has corneal disease, they should inform their doctor, as timolol may cause dry eyes.
If the patient is scheduled for surgery, they should inform their doctor about using Cusimolol 0.5%, as timolol may affect the effectiveness of certain medicines used during anesthesia.
Children and adolescents
Timolol in the form of eye drops should be used in young patients with caution. In newborns, infants, and younger children, timolol should be used with special caution. If coughing, wheezing, abnormal breathing, or unusual pauses in breathing (apnea) occur, the use of the medicine should be stopped immediately and the doctor should be contacted without delay. A portable apnea monitoring device may be helpful. Timolol in the form of eye drops has been studied in infants and children aged 12 days to 5 years with increased intraocular pressure diagnosed as glaucoma. For further information, the patient should talk to their doctor.
Cusimolol 0.5% and other medicines
The patient should tell their doctor or pharmacist about all medicines they are currently taking or have recently taken, as well as any medicines they plan to take. Cusimolol 0.5% may affect the action of other medicines used at the same time, including eye drops used to treat glaucoma. The patient should consult their doctor, especially if they are taking any of the following medicines:
- calcium channel blockers (e.g., nifedipine, verapamil, or diltiazem), usually used to treat high blood pressure, angina pectoris, heart rhythm disorders, or Raynaud's syndrome;
- digoxin, a medicine used to treat heart failure or heart rhythm disorders;
- medicines that counteract the action of catecholamines (e.g., rauwolfia alkaloids/reserpine),
- pressor amines (e.g., adrenaline), used to treat severe allergic reactions,
- quinidine, a medicine used to treat heart rhythm disorders or certain types of malaria;
- clonidine, a medicine used to treat high blood pressure;
- other beta-adrenergic blocking agents (e.g., other timolol-containing medicines, given orally or in the form of eye drops), belonging to the same group as Cusimolol 0.5% and may have an additive effect with it;
- medicines used in diabetes;
- antidepressants, such as fluoxetine and paroxetine;
- antiarrhythmic medicines (including amiodarone);
- guanethidine (used to treat high blood pressure);
- parasympathomimetic medicines.
If the patient is using other eye drops or ointments, they should leave an interval of at least 5 minutes between administrations of the next medicines. Eye ointments should be used last.
Pregnancy, breastfeeding, and fertility
If the patient is pregnant or breastfeeding, thinks they may be pregnant, or plans to have a child, they should consult their doctor or pharmacist before using this medicine. Cusimolol 0.5% should not be used during pregnancy unless the doctor considers it necessary. Cusimolol 0.5% should not be used during breastfeeding. Timolol may pass into breast milk.
Driving and using machines
Cusimolol 0.5% eye drops, solution, have a minor effect on the ability to drive and use machines. However, while using Cusimolol 0.5%, side effects such as hallucinations, dizziness, vision disturbances, fatigue may occur, which can impair the patient's ability to drive and use machines (see section 4). Until these symptoms disappear, the patient should not drive vehicles or operate machines.
Cusimolol 0.5% contains benzalkonium chloride
The medicine contains 0.5 mg of benzalkonium chloride in every 5 milliliters of solution, which corresponds to 0.1 mg/ml. Benzalkonium chloride may be absorbed by soft contact lenses and change their color. The patient should remove their contact lenses before instilling the drops and wait at least 15 minutes before putting them back on. Benzalkonium chloride may also cause eye irritation, especially in people with dry eye syndrome or corneal disorders (the transparent layer at the front of the eye). If abnormal sensations in the eye, stinging, or eye pain occur after using the medicine, the patient should contact their doctor.
Cusimolol 0.5% contains phosphates
The medicine contains 47 mg of phosphates in every 5 milliliters of solution, which corresponds to 9.4 mg/ml. In patients with severe damage to the transparent, front part of the eye (cornea), phosphates may rarely cause corneal clouding due to calcium deposition during treatment.
3. How to use Cusimolol 0.5%
This medicine should always be used as directed by the doctor. If the patient has any doubts, they should consult their doctor or pharmacist. Cusimolol 0.5% should only be used for eye drops. If the protective cap is loose after removing the cap, it should be removed before using the medicine. The patient should not start treatment with Cusimolol 0.5%. Treatment should be started with 0.25% timolol eye drops. The recommended initial dose is one drop of 0.25% timolol solution twice a day (morning and evening). If the response to treatment is not satisfactory, the doctor may increase the dose to 1 drop of 0.5% timolol solution in the affected eye, twice a day - then the patient should start using Cusimolol 0.5%. If satisfactory control of intraocular pressure is maintained, the doctor may decide to use the medicine once a day. To achieve a stronger therapeutic effect, the doctor may decide to use Cusimolol 0.5% with other medicines that lower intraocular pressure. However, it is not recommended to use Cusimolol 0.5% with topical beta-adrenergic blocking agents. Switching from another glaucoma medicineIf the doctor decides to switch from another glaucoma medicine to timolol solution, the following dosing is usually used. If the patient is using another topical beta-adrenergic blocking agent, they should stop using it after the full dose the previous day and start treatment with 0.25% timolol solution the next day, administering 1 drop of 0.25% solution twice a day in the affected eye. If the response to treatment is not satisfactory, the doctor may increase the dose to 1 drop of 0.5% timolol solution twice a day - then Cusimolol 0.5% can be used. When switching from a single-component glaucoma medicine that is not a topical beta-adrenergic blocking agent, the patient should continue using it on the same day and start using 0.25% timolol solution, administering 1 drop of 0.25% solution twice a day in the affected eye. The next day, they should stop using the previously used medicine and administer 1 drop of 0.25% timolol solution twice a day in the affected eye. If the doctor considers it necessary to use higher doses - then Cusimolol 0.5% can be used at a dose of 1 drop twice a day. If the patient feels that the action of the medicine is too strong or too weak, they should consult their doctor.
Use in children and adolescents
DosageThe use of timolol in children must be preceded by a thorough medical examination. The doctor will carefully assess the possible risks and benefits, considering the decision to treat with timolol. If the expected benefits outweigh the possible risks, it is recommended to use the medicine once a day, at the lowest available concentration. In the case of use in children, a concentration of the active substance of 0.1% should be sufficient to control intraocular pressure. If satisfactory control of intraocular pressure is not achieved at this dosage, it may be necessary to administer the medicine twice a day at 12-hour intervals. Patients, especially newborns, should be closely monitored for 1 to 2 hours after administration of the first dose and should be under close monitoring for adverse reactions until surgery is performed. Method of administrationOnly one drop of the medicine should be administered at a time (at one time of dosing). After instillation, the patient should keep their eyes closed for 2 minutes and press the corner of their eye near their nose with their finger to prevent the medicine from entering the body. Duration of treatmentIn children, the medicine is intended for temporary treatment.
Method of administration
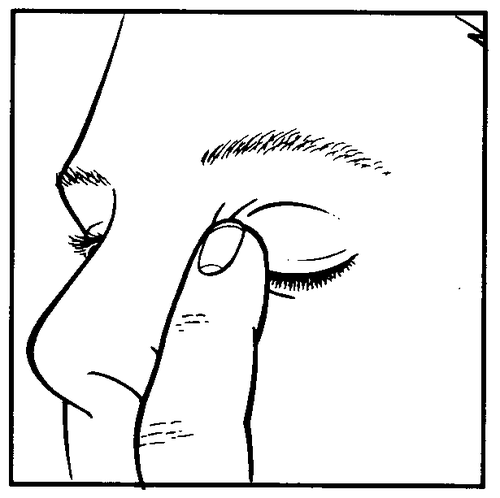
1 2 3
- Prepare the bottle of Cusimolol 0.5% and a mirror.
- Wash your hands.
- Take the bottle in your hand and unscrew the cap.
- Hold the bottle with the nozzle down, holding it with your thumb and index finger.
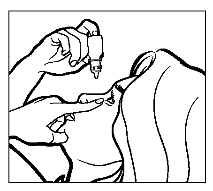
- Tilt your head back. With a clean finger, pull down the lower eyelid to create a "pocket" between the eyeball and the eyelid; the drop should fall into it (drawing 1).
- Bring the tip of the bottle close to the eye. To make it easier, you can use a mirror.
- Do not touch the tip of the dropper bottle to the eye or eyelid, the area around the eyes, or other surfaces, as this may cause contamination (infection) of the medicine in the bottle.
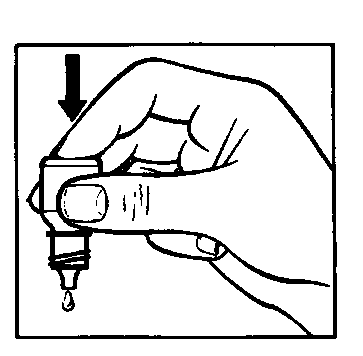
- Gently press the bottom of the bottle to cause one drop of Cusimolol 0.5% to flow out.
- Do not squeeze the bottle; it is designed so that gentle pressure on the bottom is enough to cause a drop to flow out (drawing 2).
- If the medicine is to be used in both eyes, repeat the above steps for the second eye.
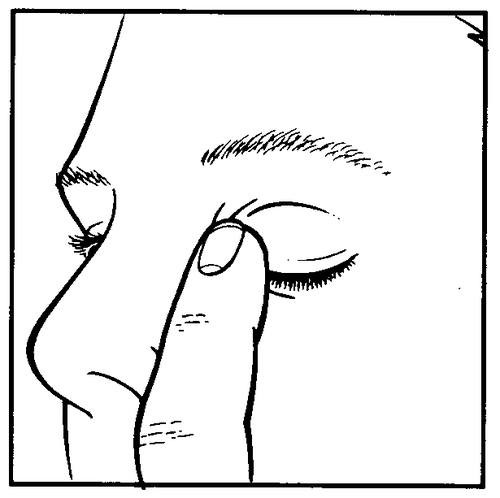
- After instilling the medicine, keep the eye closed and gently press the corner of the eye near the nose with your finger (drawing 3) for 2 minutes. This action prevents timolol from entering the body.
- Immediately after use, tighten the bottle cap tightly.
- Before opening a new bottle, the contents of the previously opened bottle should be used up.
If the drop does not get into the eye, the patient should try again to instill the medicine.
Using more than the recommended dose of Cusimolol 0.5%
If the patient uses more Cusimolol 0.5% than recommended, they should rinse their eyes with lukewarm water and contact their doctor immediately. The patient should not instill more drops in the eye until the next scheduled dose. Possible symptoms of overdose include slowed heart rate, decreased blood pressure, heart failure, and breathing difficulties.
Missing a dose of Cusimolol 0.5%
If the patient forgets to use Cusimolol 0.5%, they should continue treatment by administering the next dose of the medicine according to the dosing schedule. If there is not much time left before the next dose, the patient should skip the missed dose and continue treatment according to the recommended dosing schedule. The patient should not use a double dose to make up for the missed dose.
Stopping treatment with Cusimolol 0.5%
If the patient stops using Cusimolol 0.5%, it may cause a dangerous increase in intraocular pressure in the eye. The patient should not stop using Cusimolol 0.5% without their doctor's advice. If the patient has any further doubts about using this medicine, they should consult their doctor or pharmacist.
4. Possible side effects
Like all medicines, Cusimolol 0.5% can cause side effects, although not everybody gets them. If the patient experiences any side effects, including any side effects not listed in this leaflet, they should tell their doctor or pharmacist as soon as possible. In the event of severe side effects (e.g., allergic reactions), the patient should not instill the next dose of the medicine. They should contact their doctor or the Emergency Department of the nearest hospital immediately. The following side effects have been reported with the use of Cusimolol 0.5%: Common side effects(may occur in 1 to 10 people in 100):Eye-related effects: blurred vision, eye pain, eye irritation, eye redness Uncommon side effects(may occur in 1 to 10 people in 1,000):Eye-related effects: corneal ulcers (damage to the front surface of the eyeball), inflammation of the eye surface with or without damage to the eye surface, uveitis, conjunctivitis, blepharitis, decreased visual acuity, sensitivity to light, dry eye, increased tear production, eye discharge, eye itching, eyelid bumps, eye inflammation, eyelid swelling, eye discomfort General effects: asthma, bronchitis, shallow breathing, headache, taste disturbances, slowed heart rate, fatigue, low blood pressure Rare side effects(may occur in 1 to 10 people in 10,000):Eye-related effects: double vision, eye strain, eyelid rash, uveitis, visual impairment, eyelid drooping (causing the eye to remain half-closed), eyelid swelling, eye itching, eye redness, corneal clouding General effects: depression, reduced blood flow to the brain, dizziness, migraine, heart attack, high blood pressure, swelling (fluid retention), changes in heart rate or rhythm, heart failure, arrhythmias, heart attack, low blood pressure The following side effects have been reported during the post-marketing period (frequency not known):
- Eye-related effects: retinal detachment (separation of the layer under the retina containing blood vessels) after filtration surgery, which can cause vision disturbances; eyelid ptosis (drooping of the upper eyelid, causing the eye to remain half-closed);
- General effects: swelling in the limbs or face, which can cause respiratory distress and difficulty swallowing or breathing, allergy, low blood sugar, hallucinations, insomnia, memory loss;
As with other medicines used topically in the eye, timolol is absorbed into the systemic circulation. This may cause similar side effects as oral or intravenous beta-adrenergic blocking agents. The frequency of systemic side effects after topical administration to the eye is lower than, for example, after oral or intravenous administration. The reported side effects include effects in the entire group of beta-adrenergic blocking agents used in the eye:
- Systemic lupus erythematosus, generalized allergic reactions, including swelling in the limbs or face, which can cause respiratory distress and difficulty swallowing or breathing, urticaria or pruritic rash, local and generalized rash, itching, severe, sudden, life-threatening allergic reactions;
- Low blood sugar;
- Insomnia, depression, nightmares, memory loss;
- Fainting, stroke, reduced blood flow to the brain, worsening of myasthenia symptoms (muscle disease), dizziness, sensory disturbances, such as numbness and tingling, and headache;
- Eye irritation symptoms (e.g., burning, stinging, itching, tearing, redness), blepharitis, corneal ulcers, decreased corneal sensitivity, dry eyes, corneal clouding, eyelid ptosis (drooping of the upper eyelid, causing the eye to remain half-closed), double vision;
- Slowed heart rate, chest pain, palpitations, swelling (fluid retention), changes in heart rate or rhythm, heart failure, arrhythmias, heart attack, low blood pressure;
- Raynaud's syndrome (vasospasm in the hands, leading to poor circulation), cold hands and feet syndrome;
- Bronchospasm (mainly in patients with pre-existing disease), breathing difficulties, cough;
- Taste disturbances, nausea, gastrointestinal discomfort, diarrhea, dry mouth, abdominal pain, vomiting;
- Hair loss, skin rash with a white-silver color (psoriasis-like changes) or exacerbation of psoriasis, skin rash;
- Muscle pain not caused by physical activity;
- Sexual dysfunction, decreased libido;
- Muscle weakness/feeling of fatigue.
Reporting side effects
If the patient experiences any side effects, including any side effects not listed in this leaflet, they should tell their doctor or pharmacist. Side effects can be reported directly to the Department of Adverse Reaction Monitoring of Medicinal Products, Medical Devices, and Biocides, Urząd Rejestracji Produktów Leczniczych, Wyrobów Medycznych i Produktów Biobójczych, Al. Jerozolimskie 181C, 02-222 Warsaw, Tel.: +48 22 49 21 301, Fax: +48 22 49 21 309, website: https://smz.ezdrowie.gov.pl
5. How to store Cusimolol 0.5%
The medicine should be stored out of sight and reach of children. The medicine should be stored at a temperature below 25°C. Store in the outer packaging to protect from light. The medicine can be used for 28 days after the first opening of the bottle. Do not use this medicine after the expiry date stated on the carton and bottle. Medicines should not be disposed of via wastewater or household waste. The patient should ask their pharmacist how to dispose of medicines that are no longer needed. This will help protect the environment.
6. Contents of the packaging and other information
What does Cusimolol 0.5% contain
- The active substance of the medicine is timolol (in the form of maleic acid timolol) in an amount of 5 mg in 1 ml
- The other ingredients are: sodium dihydrogen phosphate monohydrate, disodium phosphate dodecahydrate, benzalkonium chloride, sodium chloride, purified water
What Cusimolol 0.5% looks like and what the pack contains
A bottle containing 5 ml of solution, placed in a cardboard box.
Marketing authorization holder
Immedica Pharma AB, Solnavägen 3H, SE-113 63 Stockholm, Sweden
Manufacturer
Siegfried El Masnou, S.A., c/Camil Fabra, 58, 08320 El Masnou (Barcelona), Spain
Date of last revision of the leaflet: 10/2022
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- ImporterSIEGFRIED El Masnou, S.A.
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to Cusimolol 0,5%Dosage form: Drops, 2.5 mg/mlActive substance: timololManufacturer: Warszawskie Zakłady Farmaceutyczne POLFA S.A.Prescription requiredDosage form: Drops, 5 mg/mlActive substance: timololManufacturer: Warszawskie Zakłady Farmaceutyczne POLFA S.A.Prescription requiredDosage form: Drops, 5 mg/mlActive substance: timololManufacturer: Ursapharm Arzneimittel GmbHPrescription required
Alternatives to Cusimolol 0,5% in other countries
The best alternatives with the same active ingredient and therapeutic effect.
Alternative to Cusimolol 0,5% in Spain
Alternative to Cusimolol 0,5% in Ukraine
Online doctors for Cusimolol 0,5%
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for Cusimolol 0,5% – subject to medical assessment and local rules.














