
Timo-comod 0,5 %

Ask a doctor about a prescription for Timo-comod 0,5 %

How to use Timo-comod 0,5 %
1. What is Timo-COMOD 0.5% and what is it used for
Timo-COMOD 0.5% eye drops are a solution. Timo-COMOD 0.5% contains the active substance maleate timolol, which belongs to the group of beta-adrenergic blocking agents. The action of the medicine is to reduce intraocular pressure (intraocular pressure).
Timo-COMOD 0.5% is indicated for the treatment of elevated intraocular pressure in conditions such as ocular hypertension, chronic open-angle glaucoma, including glaucoma in patients with aphakic eyes.
2. Important information before using Timo-COMOD 0.5%
When not to use Timo-COMOD 0.5%
- if the patient is hypersensitive (allergic) to the active substance, beta-adrenergic blockers, or any of the other ingredients of this medicine (listed in section 6).
- in patients with current or history of bronchial asthma;
- in patients with severe chronic obstructive pulmonary disease;
- in patients with sick sinus syndrome
- in patients with sinus bradycardia (too slow heart rhythm);
- in patients with second- and third-degree atrioventricular block not controlled by a pacemaker;
- in patients with overt heart failure;
- in patients with cardiogenic shock;
- in patients with severe allergic rhinitis;
- in patients with corneal degeneration.
Edition 07/2024
URSAPHARM * Timo-COMOD 0.5%, eye drops * MA-No. 9316 *
Application for variation
Warnings and precautions
Before starting treatment with this medicine, the patient should discuss with their doctor if they have or have had the following conditions:
- coronary heart disease (symptoms may include chest pain or tightness, shortness of breath, or choking), heart failure, low blood pressure, Prinzmetal's angina. In these patients, the doctor will consider using other medicines instead of Timo-COMOD 0.5%.
- first-degree atrioventricular block
- heart rhythm disorders such as bradycardia (slow heart rate),
- mild or moderate chronic obstructive pulmonary disease,
- diseases related to poor blood circulation, such as Raynaud's disease or Raynaud's syndrome,
- diabetes, as timolol may mask symptoms of low blood sugar,
- hyperthyroidism, as timolol may mask its symptoms
- corneal diseases
The patient should inform their doctor before surgery that they are using Timo-COMOD 0.5%, as timolol may affect the action of certain medicines used during anesthesia.
Contact lenses should be removed before administering Timo-COMOD 0.5% and waited at least 15 minutes before reinserting them.
The medicine should be used with caution in patients with cerebral circulatory disorders. If decreased cerebral circulation is detected, the doctor will consider alternative therapy.
Like other eye drops, Timo-COMOD 0.5% may be absorbed systemically and may cause side effects similar to those observed with oral beta-adrenergic blockers.
Before starting treatment with Timo-COMOD 0.5%, heart failure should be adequately controlled. In patients with a history of severe heart disease, the doctor should monitor for signs of heart failure and perform pulse checks.
After administration of timolol, cases of adverse reactions from the respiratory and cardiovascular systems have been reported, including deaths due to bronchial spasm in patients with asthma, as well as, rarely, deaths due to heart failure.
In patients taking oral beta-adrenergic blockers with Timo-COMOD 0.5%, the risk of increased intraocular pressure and systemic side effects due to beta-adrenergic receptor blockade should be considered. Concomitant use of two topical beta-adrenergic blockers is not recommended.
Cases of skin rash and/or dry eye associated with the use of beta-adrenergic blockers have been reported.
The frequency of these adverse reactions is low, and in most cases, they disappeared after discontinuation of treatment. The doctor will consider discontinuing the medicine if the above symptoms cannot be explained by other causes. Discontinuation of beta-adrenergic blockers should be gradual and under medical supervision.
Cases of retinal detachment after administration of medicines that reduce aqueous humor production (e.g., timolol, acetazolamide) after filtration surgery have been reported.
In patients with angle-closure glaucoma, the immediate goal of treatment is to reopen the angle of filtration, which requires constriction of the pupil with a miotic agent. Timo-COMOD 0.5% has no effect or a minimal effect on pupil size. If Timo-COMOD 0.5% is administered to reduce elevated intraocular pressure in a patient with angle-closure glaucoma, a miotic agent should be used concomitantly.
Edition 07/2024
URSAPHARM * Timo-COMOD 0.5%, eye drops * MA-No. 9316 *
Application for variation
If another eye disease occurs during treatment (e.g., injury, surgery, or infection), the patient should immediately consult their doctor about continuing to use the medicine from the open multidose container. There have been reports of bacterial keratitis associated with the use of eye drops in multidose containers. These containers have been contaminated by carelessness on the part of patients, most of whom had pre-existing corneal disease or damage to the surface epithelium of the eye.
Eye drop solutions may become contaminated with bacteria if not stored and used properly, causing eye infections. Using contaminated solutions can lead to serious eye damage and vision loss.
Risk of anaphylactic reaction
Children and adolescents
Caution should be exercised when using timolol-containing solutions in children and adolescents with glaucoma. Due to the potential effect on the central nervous system, this medicinal product is not recommended for use in premature infants and newborns.
Particular caution should be exercised when using timolol in neonates, infants, and small children due to the risk of apnea and Cheyne-Stokes respiration.
If coughing, wheezing, respiratory disorders, or pathological pauses in breathing (apnea) occur, the use of the timolol-containing medicine should be discontinued immediately, and medical attention should be sought as soon as possible. A portable apnea monitor may be useful.
When using timolol-containing eye drops, only one drop should be administered at a time. After administering the eye drops, the eye should be kept closed for as long as possible (e.g., 3-5 minutes) and the inner corner of the eye should be pressed to prevent the eye drops from being absorbed into the body. Do not touchthe dropper to the eye, eyelid, or surrounding areas, as this may cause infection of the dropper.
Timo-COMOD 0.5% and other medicines
The patient should tell their doctor or pharmacist about all medicines they are currently taking or plan to take.
Note: When using other eye drops, a minimum interval of 15 minutes should be maintained between the different medicinal products.
Eye ointments should always be applied last.
No specific interaction studies have been conducted with timolol-containing eye drops and other medicinal products. Timo-COMOD 0.5% may change the action of or its action may be changed by other medicines used by the patient, including other eye drops used to treat glaucoma.
The patient should tell their doctor or pharmacist about all medicines they are currently taking or plan to take, especially if they are using:
- calcium channel blockers
- beta-adrenergic blockers
- antiarrhythmic agents (including amiodarone)
- catecholamine-releasing agents
- digitalis glycosides
- parasympathomimetics
- guanethidine
- adrenaline
- CYP2D6 inhibitors (e.g., quinidine, fluoxetine, paroxetine)
Edition 07/2024
URSAPHARM * Timo-COMOD 0.5%, eye drops * MA-No. 9316 *
Application for variation
- pilocarpine
- nifedipine
- verapamil
- diltiazem
The patient should tell their doctor if they are taking or plan to take medicines that lower blood pressure, heart medicines, or antidiabetic medicines.
Pregnancy and breastfeeding
If the patient is pregnant or breastfeeding, thinks they may be pregnant, or plans to have a child, they should consult their doctor or pharmacist before using this medicine.
Pregnancy:
Timo-COMOD 0.5% should not be used during pregnancy unless the doctor considers it necessary.
Breastfeeding:
Timo-COMOD 0.5% should not be used during breastfeeding. Timolol may pass into breast milk. Before using this medicine during breastfeeding, the patient should consult their doctor.
Driving and using machines
Even when used in the recommended dose and according to the instructions, the medicine may cause visual disturbances after administration. This may affect the ability to react quickly when driving or operating machinery.
The patient should not drive or operate machinery until these symptoms have completely resolved.
The medicine contains phosphates.
Lek Timo-COMOD 0.5% contains phosphates
The medicine contains 133.6 mg of phosphates in every 10 ml, which corresponds to 13.36 mg/ml.
In patients with severe damage to the transparent, anterior part of the eye (cornea), phosphates may rarely cause corneal clouding due to calcium deposition during treatment.
3. How to use Timo-COMOD 0.5%
This medicine should always be used as directed by the doctor. If the patient has any doubts, they should consult their doctor or pharmacist.
The recommended dose is 1 drop of Timo-COMOD 0.5% twice a day into the conjunctival sac of the affected eye.
How to administer the eye drops:
Before each use, the patient should remove the cap from the dropper.
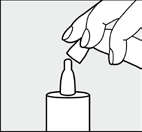
The bottle should be held with the dropper pointing down, the thumb on top of the bottle, and the index and middle fingers on the bottom of the bottle.
Note! Before first use, the patient should press the bottom of the bottle several times until the first drop of solution appears.
Edition 07/2024
URSAPHARM * Timo-COMOD 0.5%, eye drops * MA-No. 9316 *
Application for variation
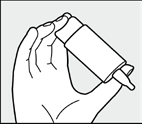
The hand holding the bottle should be supported by the other hand as shown in the picture.
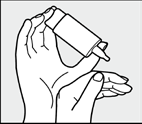
Leaning the head back slightly, the patient should pull down the lower eyelid with their free hand and quickly and firmly press the bottom of the bottle.
This will release onedrop of solution.
Thanks to the COMOD system, the drop size and delivery speed will always be the same, regardless of the pressure on the bottom of the bottle.
After administering the eye drops, the patient should slowly close their eyelid, allowing the solution to spread evenly over the eye surface.
If necessary, the patient should repeat the above steps for the other eye.
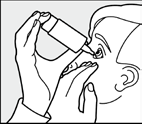
After administering Timo-COMOD 0.5%, the patient should press the inner corner of the eye (as shown in the picture) for 2 minutes.
This will prevent timolol from entering the body.

After each use, the bottle should be carefully closed with the cap, making sure the dropper tip remains dry.
The patient should avoid touching the dropper to the eye or skin of the face.
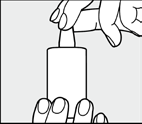
Timo-COMOD 0.5% eye drops do not contain preservatives. A special pump called the COMOD system is used. Unlike traditional eye drop containers, the patient should press the bottom of the bottle (as shown in the picture) when administering the eye drops.
If the patient feels that the effect of the medicine is too strong or too weak, they should consult their doctor.
Use in children and adolescents:
Dosage:
Before using timolol, a thorough medical examination should be performed. When considering treatment with timolol, the doctor will carefully weigh the risks and benefits. If the benefits outweigh the risks, the use of eye drops with the lowest concentration of timolol (0.1%) once a day is recommended. In children and adolescents, a timolol concentration of 0.1% should be sufficient to control intraocular pressure. If control of intraocular pressure is not achieved with this dose, it may be necessary to use the medicine twice a day at 12-hour intervals.
Patients, especially newborns, should be closely monitored for one to two hours after the first dose and strictly monitored for side effects until surgery is performed.
Method of administration:
The patient should always administer only one drop of timolol-containing eye drops at a time. After administering the eye drops, the patient should keep their eye closed for as long as possible (e.g., 3-5 minutes) and press the inner corner of the eye to prevent the eye drops from being absorbed into the body. Do not touchthe dropper to the eye, eyelid, or surrounding areas, as this may cause infection of the dropper.
Overdose of Timo-COMOD 0.5%
The patient should immediately rinse their eyes with water and consult their doctor or pharmacist.
Overdose may lead to severe hypotension, heart failure, cardiogenic shock, and bradycardia, up to cardiac arrest.
Additionally, respiratory disorders, bronchospasm, vomiting, disturbances of consciousness, generalized seizures may occur.
The doctor will use general measures, monitor vital functions, and use supportive measures if necessary in an intensive care setting.
Missed dose of Timo-COMOD 0.5%
The patient should not use a double dose to make up for a missed dose.
Discontinuation of Timo-COMOD 0.5%
Before discontinuing the medicine, the patient should consult their doctor.
If the patient has any further doubts about using this medicine, they should consult their doctor or pharmacist.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
The patient can usually continue using this medicine, unless the side effects are serious. If the patient has any doubts, they should talk to their doctor or pharmacist. If the patient experiences an allergic reaction, including rash, swelling of the face, lips, tongue, and/or throat, which may cause difficulty breathing or swallowing, or other serious side effects, they should discontinue using Timo-COMOD and immediately contact their doctor or the emergency department of the nearest hospital.
Reporting side effects
If the patient experiences any side effects, including any side effects not listed in the leaflet, they should tell their doctor or pharmacist, or nurse. Side effects can be reported directly to the Department of Monitoring of Adverse Reactions to Medicinal Products of the Office for Registration of Medicinal Products, Medical Devices, and Biocidal Products:
Jerozolimskie Avenue 181C, 02-222 Warsaw, phone: 22 49-21-301, fax: 22 49-21-309, website: https://smz.ezdrowie.gov.pl .
Side effects can also be reported to the marketing authorization holder.
By reporting side effects, more information can be collected on the safety of the medicine.
Edition 07/2024
URSAPHARM * Timo-COMOD 0.5%, eye drops * MA-No. 9316 *
Application for variation
5. How to store Timo-COMOD 0.5%
The medicine should be stored out of sight and reach of children.
Do not use this medicine after the expiry date stated on the label. The expiry date refers to the last day of the month.
Store in a temperature below 25°C.
Shelf life after first opening the bottle: 12 weeks.
Medicines should not be disposed of via wastewater or household waste. The patient should ask their pharmacist how to dispose of medicines that are no longer needed. This will help protect the environment.
6. Contents of the packaging and other information
What does Timo-COMOD 0.5% contain
The active substance of the medicine is timolol.
1 ml of eye drop solution contains 5.0 mg of timolol (in the form of timolol maleate 6.84 mg)
The other ingredients are: sodium dihydrogen phosphate dihydrate, disodium phosphate dodecahydrate, water for injections.
What does Timo-COMOD 0.5% look like and what does the pack contain
Timo-COMOD 0.5% is a clear, colorless eye drop solution.
The carton contains 1 bottle with a dispenser (COMOD system) containing 10 ml of eye drop solution.
Marketing authorization holder and manufacturer
Marketing authorization holder:
URSAPHARM Poland sp. z o.o., Wybrzeże Gdyńskie 27, 01-531 Warsaw, Poland
Phone: 22 732 07 90
Fax: 22 732 07 99
e-mail: [email protected]
Manufacturer:
URSAPHARM Arzneimittel GmbH, Industriestraße, 66129 Saarbrücken, Germany
Date of last revision of the leaflet
Edition 07/2024
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- ImporterUrsapharm Arzneimittel GmbH
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to Timo-comod 0,5 %Dosage form: Drops, 5 mg/mlActive substance: timololManufacturer: SIEGFRIED El Masnou, S.A.Prescription requiredDosage form: Drops, 2.5 mg/mlActive substance: timololManufacturer: Warszawskie Zakłady Farmaceutyczne POLFA S.A.Prescription requiredDosage form: Drops, 5 mg/mlActive substance: timololManufacturer: Warszawskie Zakłady Farmaceutyczne POLFA S.A.Prescription required
Alternatives to Timo-comod 0,5 % in other countries
The best alternatives with the same active ingredient and therapeutic effect.
Alternative to Timo-comod 0,5 % in Spain
Alternative to Timo-comod 0,5 % in Ukraine
Online doctors for Timo-comod 0,5 %
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for Timo-comod 0,5 % – subject to medical assessment and local rules.














