

Tafen Nasal 32 mcg

Ask a doctor about a prescription for Tafen Nasal 32 mcg

How to use Tafen Nasal 32 mcg
Leaflet attached to the packaging: patient information
Tafen Nasal 32 μg
32 micrograms/dose, nasal spray, suspension
Budesonide
Read the leaflet carefully before using the medicine, as it contains important information for the patient.
- The leaflet should be kept in case it needs to be read again.
- In case of any doubts, the doctor or pharmacist should be consulted.
- This medicine has been prescribed to a specific person. It should not be given to others. The medicine may harm another person, even if the symptoms of their illness are the same.
- If the patient experiences any side effects, including any side effects not listed in this leaflet, they should tell their doctor. See section 4.
Table of contents of the leaflet
- 1. What is Tafen Nasal 32 μg and what is it used for
- 2. Important information before using Tafen Nasal 32 μg
- 3. How to use Tafen Nasal 32 μg
- 4. Possible side effects
- 5. How to store Tafen Nasal 32 μg
- 6. Contents of the packaging and other information
1. What is Tafen Nasal 32 μg and what is it used for
Tafen Nasal 32 μg contains budesonide, a synthetic corticosteroid. Corticosteroids are a group of medicines that help fight inflammation.
Tafen Nasal 32 μg is used for:
treatment and prevention of allergy symptoms, such as hay fever (caused by grass pollen) and symptoms of year-round, allergic rhinitis (chronic rhinitis), caused by house dust, in adults and children over 6 years old,
treatment of nasal polyp symptoms (small growths on the nasal mucosa) in adults.
2. Important information before using Tafen Nasal 32 μg
When not to use Tafen Nasal 32 μg
- if the patient is allergic to budesonide or any other ingredient of this medicine (listed in section 6).
- if the patient has bleeding disorders or nosebleeds.
- if the patient has blisters around the mouth, nose, or eyes related to herpes.
Warnings and precautions
Before starting treatment with Tafen Nasal 32 μg, the doctor or pharmacist should be consulted if:
- the patient is taking other corticosteroid medicines, such as tablets or injectable medicines, they should not stop taking them suddenly.
- the medicine is used continuously for a long time, the doctor will examine the nasal mucosa at least every 6 months;
- the medicine was used in higher doses than recommended, the doctor may prescribe steroid tablets for use during stressful periods (such as infection) or before surgery;
- the patient has nasal mucosa ulcers, using Tafen Nasal 32 μg is not recommended;
- the patient has infectious blisters (herpes) around the mouth, nose, or eyes;
- if symptoms of infection occur, such as persistent fever;
- if the patient has diabetes, corticosteroids may increase blood sugar levels;
- the patient has nosebleeds, using Tafen Nasal 32 μg is not recommended;
- the patient has recently undergone nose surgery or injury and has not yet fully recovered, using Tafen Nasal 32 μg is not recommended;
- the patient has a bacterial, viral, or fungal infection of the nose; Tafen Nasal 32 μg can only be used if medicines for the treatment of these infections are also prescribed;
- if the patient has been in contact with someone with tuberculosis, measles, or chickenpox
- the patient has liver function disorders, as the budesonide concentration in the body may increase; the doctor may recommend liver function tests and, depending on the result, reduce the dose of the medicine;
- the patient has adrenal function disorders and the doctor has changed their medication to a nasal spray;
- the doctor has diagnosed the patient with a respiratory tract infection or pulmonary tuberculosis - these are infections that affect lung function;
- in rare cases, locally administered corticosteroids may cause side effects that affect the whole body. These are most often dose-dependent, duration of treatment, additional concurrent or previous corticosteroid use, and individual factors. Nasally administered corticosteroids may cause the following side effects: Cushing's syndrome, Cushingoid features, adrenal insufficiency, growth retardation in children and adolescents, cataracts, and increased intraocular pressure (glaucoma). Less common are psychological symptoms and behavioral changes, including excessive psychomotor activity, sleep disturbances, anxiety, depression, or aggression (especially in children).
If the patient experiences blurred vision or other vision disturbances, or if any symptoms occur, persist, or worsen during treatment with this medicine, they should consult their doctor.
To ensure the medicine is effective, the nostrils must be cleaned. Therefore, before taking a dose, the nose should be blown.
The effect of the medicine on symptoms may only appear after a few days of treatment.
If nasal congestion (feeling of a blocked nose) persists despite starting treatment, the doctor should be consulted to re-evaluate the treatment.
Athletes should be cautious, as this medicine contains an active ingredient that may cause a positive result in doping tests.
Children and adolescents
The long-term effects of locally administered corticosteroids in children are not fully known.
If the patient is a child receiving large doses of the medicine for a long time, the doctor will regularly monitor their growth.
Tafen Nasal 32 μg and other medicines
This medicine may affect the action of other medicines or be affected by other medicines.
The doctor or pharmacist should be told about all medicines the patient is currently taking or has recently taken, as well as any medicines they plan to take, including those available without a prescription.
The doctor should be informed about the use of:
medicines used to treat fungal infections (such as ketoconazole, itraconazole, posaconazole, or voriconazole),
cyclosporine, an immunosuppressant medicine used, for example, in transplants,
ethinylestradiol, a medicine used to prevent pregnancy,
antibacterial medicines (such as erythromycin, clarithromycin, telithromycin, ciprofloxacin, levofloxacin, troleandomycin, and others),
boceprevir (a medicine used to treat hepatitis C, a liver disease caused by hepatitis C virus),
non-steroidal anti-inflammatory medicines (NSAIDs) (e.g., containing acetylsalicylic acid), used to reduce pain, fever, and inflammation,
heparin and oral anticoagulants (medicines that prevent blood clots),
antiepileptic medicines used to treat epilepsy (such as carbamazepine, phenobarbital, phenytoin, sodium valproate),
cobimetinib (a medicine used to treat certain types of skin and mucous membrane cancer, called melanoma),
medicines that affect liver metabolism, called enzyme inducers (such as St. John's wort),
medicines containing activated carbon, used to relieve stomach pain, reflux, or gas,
Some medicines may enhance the effect of Tafen Nasal 32 μg, and the doctor may want to closely monitor the patient's condition when taking such medicines (including some HIV medicines: saquinavir, atazanavir, indinavir, nelfinavir, ritonavir, cobicistat).
Pregnancy and breastfeeding
If the patient is pregnant or breastfeeding, thinks they may be pregnant, or plans to have a child, they should consult their doctor or pharmacist before using this medicine.
Do not useTafen Nasal 32 μg during pregnancy, unless previously agreed with the doctor.
If the patient becomes pregnant during treatment, they should consult their doctor.
Budesonide passes into breast milk, but at the usual doses, it is unlikely to affect the baby. Breastfeeding mothers may useTafen Nasal 32 μg, but only if the doctor believes the benefits of treatment for the mother outweigh the risk to the breastfed baby. If the woman is breastfeeding, she should inform her doctor immediately.
It is recommended to avoid breastfeeding during long-term treatment.
Driving and using machines
Tafen Nasal 32 μg may cause blurred vision. If this occurs, the patient should not drive or operate machinery.
Tafen Nasal 32 μg contains potassium sorbate
Tafen Nasal 32 μg contains potassium sorbate. It may cause skin or mucous membrane irritation (e.g., nasal mucosa irritation).
3. How to use Tafen Nasal 32 μg
This medicine should always be used as directed by the doctor or pharmacist. If in doubt, the doctor or pharmacist should be consulted.
Tafen Nasal 32 μg is intended for nasal administration, as described below.
Dosage
The dose should be adjusted to the patient's needs. The smallest dose that relieves symptoms should be used.
Allergic rhinitis
Initial dose
Adults, adolescents (from 12 years old), and children over 6 years old:
The recommended initial dose of the medicine is 8 dosesof 32 micrograms/dose aerosolTafen Nasal 32 μg (256 micrograms) per day.
The medicine can be used:
once a day, 4 doses of aerosol into each nostril in the morning
or
twice a day, 2 doses of aerosol into each nostril in the morning and 2 doses of aerosol into each nostril in the evening.
Children should be treated under adult supervision.
If the medicine is used in children for more than 2 months in a year, a pediatrician's consultation is necessary.
It is best to start using the medicine 14 days before the expected onset of symptoms.
For example, if the patient has hay fever, treatment should start about 2 weeks before the pollen season and stop after the end of the pollen season.
Maintenance dose
The effect of the medicine occurs after 7 to 14 days. After this time, the doctor may reduce the dose.
Nasal polyps
Adults:
The recommended initial dose of the medicine is 8 dosesof 32 micrograms/dose aerosolTafen Nasal 32 μg (256 micrograms) per day.
The medicine can be used:
once a day, 4 doses of aerosol into each nostril in the morning
or
twice a day, 2 doses of aerosol into each nostril in the morning and 2 doses of aerosol into each nostril in the evening.
After the effect is achieved, the smallest dose of the medicine that relieves symptoms should be used.
Using more than the recommended 8 dosesof Tafen Nasal 32 μg per daywill not increasethe effectiveness of the medicine.
Duration of treatment
The doctor will inform the patient how long the treatment with Tafen Nasal 32 μg will last. The medicine must be used regularly; otherwise, it will not be effective. Treatment should not be stopped without the doctor's advice, even if the patient feels better.
If using the medicine does not bring immediate improvement, the patient should continue to take the medicine regularly, as the therapeutic effect may occur after a few days.
Method of administration
- 1. If necessary, the nose should be gently blown to clean the nostrils.
- 2. Shake the bottle. Remove the protective cap (Fig. 1).
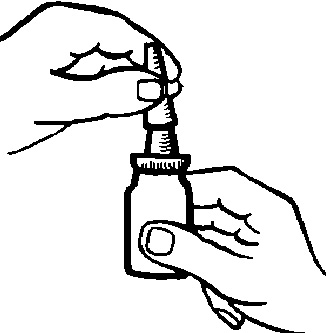
Fig. 1
- 3. Hold the bottle as shown in Figure 2. Before first use, the pump must be primed (i.e., filled with medicine). This is done by moving the piston up and down several times (5-10 times), spraying the medicine into the air until a visible mist is produced. Priming is sufficient for about 24 hours. If more time passes before the next dose, the pump should be re-primed. If the breaks in using Tafen Nasal 32 μg are shorter, it is enough to spray the medicine into the air once.
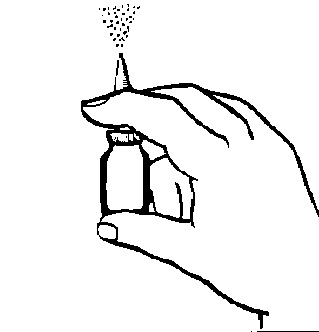
Fig. 2
- 4. Insert the applicator tip into the nostril, as shown in Figure 3, and spray once (or more, if advised by the doctor). The same procedure should be used to administer the aerosol to the other nostril. Note: During spraying, it is not necessary to inhale.
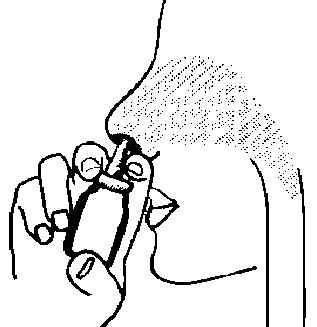
Fig. 3
- 5. Wipe the applicator with a clean tissue and replace the protective cap.
- 6. The bottle should be stored upright.
Cleaning the applicator
The plastic applicator should be cleaned at regular intervals and every time the medicine cannot be sprayed properly. In this case, first check if the pump is primed (see above). If the pump still does not work after re-priming, the applicator should be cleaned according to the following instructions:
- Remove the plastic applicator using a clean tissue and wash it in warm (not hot) water.
- Rinse the applicator thoroughly, dry, and reattach it to the bottle.
- Never use a needle or other sharp object to unclog the applicator.
- After cleaning the applicator, the pump must be primed (filled with medicine) before reusing.
Using a higher dose of Tafen Nasal 32 μg than recommended
It is essential to use the medicine as directed by the doctor. The patient should use exactly the amount of medicine prescribed by the doctor. Using a higher or lower dose of the medicine may worsen the symptoms of the disease.
If a higher dose of Tafen Nasal 32 μg than recommended is used, the patient should continue to use the medicine according to the usual dosage schedule. No health problems are expected.
However, if more than the recommended 8 dosesof Tafen Nasal 32 μg per dayare used for more than a month, the doctor should be consulted immediately.
Missing a dose of Tafen Nasal 32 μg
If a dose is missed, it should be taken as soon as possible, and then the usual dosage schedule should be resumed. A higher dose than recommended should not be used to make up for the missed dose.
In case of further doubts about using this medicine, the doctor or pharmacist should be consulted.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
The medicine usually only treats symptoms that occur in the nose (e.g., congestion or discharge).
If the patient previously received steroids in the form of tablets or injectable steroids, but the doctor has now prescribed Tafen Nasal 32 μg instead, some other symptoms may worsen (such as redness and itching of the eyes). In this case, the doctor will treat these symptoms separately.
After using this medicine, allergic reactions have been reported: hives, itching, skin rash, angioedema (swelling of the face, lips, tongue, and/or throat) with difficulty breathing and swallowing, and a general feeling of discomfort.
If these symptoms occur after using Tafen Nasal 32 μg, the patient should stop using the medicine and consult their doctor immediately.
During treatment with Tafen Nasal 32 μg, the following side effects may occur:
Common side effects (may occur in less than 1 in 10 people)
These effects may occur immediately after using the medicine:
occasional sneezing, dryness, or stinging in the nose
small amount of bloody discharge from the nose
nosebleeds (immediately after administration)
pain in the mouth and/or throat
Uncommon side effects (may occur in less than 1 in 100 people)
redness of the skin
skin irritation
muscle cramps
Rare side effects (may occur in less than 1 in 1000 people)
anaphylactic reaction
bone fragility (after long-term use of the medicine)
bruises or bruises
increased eye pressure
blurred vision
sores or painful ulcers in the nose
perforation of the nasal septum (nasal septum)
voice disorders
slower growth rate in children and adolescents, especially after using large doses for a long time
adrenal insufficiency. This can cause loss of appetite, abdominal pain, weight loss, nausea, headaches, vomiting, changes in consciousness, decreased blood sugar levels, and seizures. Situations that can potentially trigger an adrenal crisis include injury, infection, surgery, or sudden reduction in dose.
If such symptoms occur, the doctor should be consulted immediately.
Unknown frequency (cannot be estimated from available data)
glaucoma (eye pain, redness of the eye, blurred vision)
cataract (clouding of the lens of the eye)
headache
thrush of the nose and mouth and throat (difficulty eating and/or swallowing, thrush (white spots in the mouth, throat, or on the tongue))
Thrush:
During long-term treatment, thrush of the nose (fungal infection) may occasionally occur.
The doctor should be consulted to discuss appropriate treatment. In this case, treatment should be discontinued until the infection is cured.
Systemic effects:
Nasal corticosteroids in high doses and used for a long time may have a systemic effect.
The following symptoms may occur:
- hypercorticism (occurring with symptoms such as weight gain, moon face, fatigue, and/or increased abdominal circumference)
The potassium sorbate ingredient in this medicine may cause local skin or mucous membrane irritation, such as nasal mucosa irritation.
Reporting side effects
If any side effects occur, including any side effects not listed in this leaflet, the doctor or pharmacist should be informed. Side effects can be reported directly to the Department of Adverse Reaction Monitoring of Medicinal Products, Medical Devices, and Biocidal Products:
Al. Jerozolimskie 181C, 02-222 Warsaw,
tel.: +48 22 49 21 301, fax: +48 22 49 21 309, website: https://smz.ezdrowie.gov.pl
Side effects can also be reported to the marketing authorization holder.
Reporting side effects can help gather more information on the safety of this medicine.
5. How to store Tafen Nasal 32 μg
The medicine should be stored out of sight and reach of children.
Do not use this medicine after the expiry date stated on the packaging after EXP.
The expiry date refers to the last day of the specified month.
Do not store above 30°C.
Do not freeze.
After 3 months from opening, the bottle with the remaining suspension should be discarded.
Medicines should not be disposed of via wastewater or household waste. The pharmacist should be asked how to dispose of medicines that are no longer needed. This will help protect the environment.
6. Contents of the packaging and other information
What does Tafen Nasal 32 μg contain
The active substance of the medicine is budesonide. One dose of 0.05 ml of nasal spray suspension contains 32 micrograms (μg) of budesonide.
The other ingredients are: dispersible cellulose (microcrystalline cellulose and sodium carmellose (89:11 w/w)), polysorbate 80, potassium sorbate (E 202), anhydrous glucose, disodium edetate, concentrated hydrochloric acid, ascorbic acid (E 300), water for injections.
What Tafen Nasal 32 μg looks like and what the packaging contains
Tafen Nasal 32 μg is a white or almost white, homogeneous suspension.
Tafen Nasal 32 μg is available in a brown glass bottle with a plastic spray pump and a polypropylene nasal applicator: 1 x 120 doses.
Marketing authorization holder and manufacturer
Marketing authorization holder
Sandoz GmbH
Biochemiestrasse 10
A-6250 Kundl, Austria
tel. 22 209 70 00
Manufacturer
Lek Pharmaceuticals d.d.
Verovškova 57
1526 Ljubljana, Slovenia
This medicine is authorized for use in the Member States of the European Economic Area under the following names:
Germany:
Budesonid Sandoz 32 Mikrogramm/Sprühstoß Nasenspray, Suspension
Denmark:
Budesonid "Sandoz"
Norway:
Budesonid Sandoz 32 mikrogram/dose nesespray, suspensjon
Poland:
TAFEN NASAL 32 µg
Sweden:
Desonix 32 mikrogram/dos nässpray, suspension
Date of last revision of the leaflet:11/2024
{Logo Sandoz}
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- ImporterLEK Pharmaceuticals d.d. LEK S.A. Salutas Pharma GmbH
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to Tafen Nasal 32 mcgDosage form: Aerosol, 64 mcg/measured doseActive substance: budesonideManufacturer: Lek Pharmaceuticals d.d. LEK S.A. Salutas PharmaPrescription requiredDosage form: Aerosol, 50 mcg/nasal doseActive substance: budesonideManufacturer: Lek Pharmaceuticals d.d.Prescription requiredDosage form: Aerosol, 50 mcg/doseActive substance: mometasoneManufacturer: FARMEA US Pharmacia Sp. z o.o.Prescription not required
Alternatives to Tafen Nasal 32 mcg in other countries
The best alternatives with the same active ingredient and therapeutic effect.
Alternative to Tafen Nasal 32 mcg in Ukraine
Alternative to Tafen Nasal 32 mcg in Espanha
Online doctors for Tafen Nasal 32 mcg
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for Tafen Nasal 32 mcg – subject to medical assessment and local rules.









