
BUDESONIDE NASAL ALDO-UNIÓN 100 micrograms/dose NASAL SPRAY SUSPENSION


How to use BUDESONIDE NASAL ALDO-UNIÓN 100 micrograms/dose NASAL SPRAY SUSPENSION
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
- Introduction
- What is Budesonide Nasal Aldo-Union and what is it used for
- What you need to know before taking Budesonide Nasal Aldo-Union
- How to use Budesonide Nasal Aldo-Union
- Possible side effects
- Storage of Budesonide Nasal Aldo-Union
- Keep this medicine out of the sight and reach of children.
- Package contents and additional information.
Introduction
Leaflet: Information for the patient
Budesonide Nasal Aldo-Union 100 micrograms/dose
Nasal spray suspension
Read the entire leaflet carefully before starting to use this medicine, as it contains important information for you.
- Keep this leaflet, as you may need to read it again. If you have any questions, ask your doctor, pharmacist, or nurse.
- This medicine has been prescribed to you only, and you should not give it to others, even if they have the same symptoms as you, as it may harm them.
- If you experience side effects, consult your doctor, pharmacist, or nurse, even if they are not listed in this leaflet. See section 4.
Contents of the leaflet
- What is Budesonide Nasal Aldo-Union and what is it used for
- What you need to know before taking Budesonide Nasal Aldo-Union
- How to use Budesonide Nasal Aldo-Union
- Possible side effects
- Storage of Budesonide Nasal Aldo-Union
- Package contents and additional information
1. What is Budesonide Nasal Aldo-Union and what is it used for
This medicine contains the active ingredient budesonide, which belongs to a group of medicines called glucocorticoids, and is used to reduce inflammation of the nasal mucosa (the inside of the nose).
Budesonide Nasal Aldo-Union is used to treat the symptoms of seasonal allergic rhinitis ("hay fever"), as well as perennial (year-round) and vasomotor rhinitis.
2. What you need to know before taking Budesonide Nasal Aldo-Union
Do not use Budesonide Nasal Aldo-Union
- If you are allergic (hypersensitive) to budesonide or any of the other ingredients of this medicine (listed in section 6).
Warnings and precautions
Consult your doctor or pharmacist before starting to use Budesonide Nasal Aldo-Union:
- If you have or have had tuberculosis.
- If you have untreated generalized or respiratory infections.
- If you have symptoms or signs of a localized infection in the nasal passages.
- If you have previously been treated with systemic steroids.
Contact your doctor if you experience blurred vision or other visual disturbances.
Budesonide Nasal Aldo-Union has been prescribed to you for the treatment of your current condition. Do not take this medicine for other conditions without consulting your doctor.
Avoid contact between the product and the eyes. If contact occurs, rinse your eyes immediately with plenty of water.
Children and adolescents
The administration of Budesonide Nasal Aldo-Union is not recommended in children under 6 years of age.
Children aged 6 or older should use Budesonide Nasal Aldo-Union only under the supervision of an adult to ensure correct administration and that the dose corresponds to that prescribed by the doctor. The doctor will periodically review the growth of children, as this medicine may cause growth retardation.
Using Budesonide Nasal Aldo-Union with other medicines:
Tell your doctor if you are taking or have recently taken other medicines, including those purchased without a prescription.
Some medicines may increase the effects of Budesonide Nasal Aldo-Union, so your doctor will monitor you closely if you are taking these medicines (including some for HIV: ritonavir, cobicistat).
Pregnancy, breastfeeding, and fertility
If you are pregnant or breastfeeding, or think you may be pregnant or plan to become pregnant, consult your doctor or pharmacist before using this medicine.
There is no clear evidence that this medicine harms the mother or child when administered during pregnancy or breastfeeding.
Driving and using machines
There is no indication that this medicine may affect your ability to drive.
Use in athletes
If you are an athlete, keep in mind that this medicine contains budesonide, which may result in a positive test result in doping tests.
3. How to use Budesonide Nasal Aldo-Union
Follow the administration instructions for this medicine exactly as indicated by your doctor or pharmacist. If in doubt, consult your doctor or pharmacist again.
The dose must be individualized. Your doctor will indicate the duration of treatment with Budesonide Nasal Aldo-Union; do not exceed the recommended treatment duration.
Do not share the spray with others due to the risk of infection.
Seasonal and perennial allergic rhinitis, non-allergic perennial rhinitis:
Adults: The usual dose is two applications in each nostril in the morning. It can also be administered as one application in each nostril, morning and night. Once an improvement in symptoms is achieved, your doctor may reduce the dose.
If you have seasonal allergic rhinitis ("hay fever"), you should start treatment with Budesonide Nasal Aldo-Union before the allergy season begins. This medicine does not provide immediate relief from symptoms. It may take several days of treatment with Budesonide Nasal Aldo-Union before you notice relief from symptoms (sometimes up to 2 weeks).
This medicine does not relieve eye symptoms of allergy. If you experience eye discomfort, your doctor may prescribe another medicine to relieve these symptoms.
If relief from symptoms is not achieved after 3 weeks of treatment, administration of the preparation should be discontinued. It is advisable to administer the minimum effective dose.
In patients with perennial allergic rhinitis, once adequate control of symptoms is achieved, the dose should be gradually reduced every 2-4 weeks until the expected clinical effect is maintained. If symptoms return, the dose can be increased to the initial dose and then reduced to the dose at which adequate control of symptoms was achieved.
Use in children and adolescents
The use of this medicine is not recommended in children and adolescents.
Instructions for correct administration of the preparation:
Before the first application:
- Remove the protective cap.
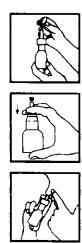 Shake the bottle-applicator combination.
Shake the bottle-applicator combination. - Press the button the necessary number of times to fill the pump mechanism and produce a correct spray.
If not used daily, a spray should be performed in the air before being used again.
Mode of use. For each application:
- Blow your nose gently
- Remove the protective cap
- Shake the bottle-applicator combination.
- Insert the applicator into one nostril, covering the other with your finger.
- Inhale and press firmly on the bottom of the bottle. Breathe through your mouth and repeat the operation.
- Repeat the same process in the other nostril.
After use, replace the protective cap.
Cleaning:
After using the spray, to keep the nozzle clean, carefully clean the nasal applicator with a tissue or a clean cloth.
If the spray does not work, the nozzle may be blocked. Never try to unblock it or make the spray hole larger with a pin or sharp object. This could cause the spray to stop working.
The nasal applicator is removed by gently pulling it upwards.
If you use more Budesonide Nasal Aldo-Union than you should:
It is important that you take your dose as indicated by your doctor. You should only use what your doctor recommends; using more or less dose can worsen your symptoms.
Consult your doctor or pharmacist, or call the Toxicology Information Service, phone: 91 5620420, indicating the medicine and the amount used.
If you forget to use Budesonide Nasal Aldo-Union:
Do not use a double dose to make up for forgotten doses. Simply apply the next dose as prescribed.
If you have any other questions about the use of this medicine, ask your doctor or pharmacist.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
The following adverse reactions have been reported, classified by system organ class and in decreasing order of frequency:
Respiratory, thoracic, and mediastinal disorders:
- Frequent(≥1/100 to <1>
- Sneezing.
- Nasal itching.
- Nasal dryness.
- Hoarseness, including voice changes
- Rare(≥1/10,000 to <1>
- Nasal bleeding.
- Throat irritation.
- Very rare(<1>
- Nasal septum perforation.
Skin and subcutaneous tissue disorders:
- Uncommon(≥1/1000, <1>
Cutaneous allergic reactions.
Eye disorders:
- Frequency not known (cannot be estimated from the available data:
- Blurred vision.
If you consider any of these side effects to be serious or if you notice any side effect not mentioned in this leaflet, tell your doctor or pharmacist.
Reporting of side effects
If you experience any side effects, consult your doctor or pharmacist, even if they are not listed in this leaflet. You can also report them directly through the Spanish Medicines and Healthcare Products Agency's (AEMPS) online platform: www.notificaRAM.es. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of Budesonide Nasal Aldo-Union
Keep this medicine out of the sight and reach of children.
Do not store above 30°C.
Keep the container in the outer packaging to protect it from light.
Do not freeze.
Replace the protective cap after using Budesonide Nasal Aldo-Union.
Do not use this medicine after the expiry date stated on the container after EXP. The expiry date is the last day of the month indicated.
Medicines should not be disposed of via wastewater or household waste. Return the containers and any unused medicines to the pharmacy's SIGRE collection point. If in doubt, ask your pharmacist how to dispose of containers and unused medicines. This will help protect the environment.
6. Package contents and additional information.
Composition of Budesonide Nasal Aldo-Union
- The active ingredient is budesonide. Each application contains 100 micrograms of budesonide (2 mg/ml).
- The other ingredients (excipients) are: anhydrous glucose, microcrystalline cellulose, and sodium carmellose, polysorbate 80, sodium edetate, potassium sorbate (E-202), hydrochloric acid, and purified water.
Appearance of the product and package contents:
Budesonide Nasal Aldo-Union is a white aqueous suspension presented in a 10 ml (200 doses) brown glass container, provided with a dosing pump and a nasal adapter.
Marketing authorization holder and manufacturer:
Laboratorio ALDO-UNIÓN, S.L.
Baronesa de Maldà, 73
08950 Esplugues de Llobregat
BARCELONA – SPAIN
Date of last revision of this leaflet: December 2024
Other sources of information
Detailed and updated information on this medicine is available on the website of the Spanish Agency for Medicines and Healthcare Products (AEMPS) http://www.aemps.gob.es/
- Country of registration
- Average pharmacy price8.12 EUR
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to BUDESONIDE NASAL ALDO-UNIÓN 100 micrograms/dose NASAL SPRAY SUSPENSIONDosage form: NASAL PRODUCT, 64 MCG/sprayActive substance: budesonideManufacturer: Laboratorio Aldo Union S.L.Prescription requiredDosage form: NASAL PRODUCT, 2 mg budesonide/ mlActive substance: budesonideManufacturer: M4 Pharma S.L.Prescription requiredDosage form: NASAL PRODUCT, 1 mg budesonide/mlActive substance: budesonideManufacturer: M4 Pharma S.L.Prescription required
Online doctors for BUDESONIDE NASAL ALDO-UNIÓN 100 micrograms/dose NASAL SPRAY SUSPENSION
Discuss questions about BUDESONIDE NASAL ALDO-UNIÓN 100 micrograms/dose NASAL SPRAY SUSPENSION, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions











