
BUDESONIDE ALCON 100 micrograms/DOSE NASAL SPRAY SUSPENSION


How to use BUDESONIDE ALCON 100 micrograms/DOSE NASAL SPRAY SUSPENSION
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
- Introduction
- What is BUDESONIDA ALCON 100 micrograms/dose and what is it used for
- What you need to know before starting to use BUDESONIDA ALCON 100 micrograms/dose
- How to use BUDESONIDA ALCON 100 micrograms/dose
- Possible side effects
- Storage of BUDESONIDA ALCON 100 micrograms/dose
- Additional Information
Introduction
Package Leaflet: Information for the User
BUDESONIDA ALCON 100 micrograms/dose nasal spray suspension
Budesonide
Read the entire package leaflet carefully before starting to use the medicine, as it contains important information for you.
- Keep this package leaflet, as you may need to read it again.
- If you have any questions, consult your doctor or pharmacist.
- This medicine has been prescribed to you only, and you should not give it to others, even if they have the same symptoms as you, as it may harm them.
- If you experience side effects, consult your doctor or pharmacist, even if they are not listed in this package leaflet.
Contents of the Package Leaflet
- What is BUDESONIDA ALCON 100 micrograms/dose and what is it used for
- What you need to know before starting to use BUDESONIDA ALCON 100 micrograms/dose
- How to use BUDESONIDA ALCON 100 micrograms/dose
- Possible side effects
- Storage of BUDESONIDA ALCON 100 micrograms/dose
- Package Contents and Additional Information
1. What is BUDESONIDA ALCON 100 micrograms/dose and what is it used for
This medicine contains budesonide, which has anti-inflammatory and antiallergic activity when applied to the nasal mucosa.
It is indicated for the relief of congestion, irritation, and localized discomfort in the nasal mucosa (rhinitis) that are a consequence of seasonal and chronic allergic processes. It is also used for the treatment of nasal polyps and their prevention after polypectomy.
2. What you need to know before starting to use BUDESONIDA ALCON 100 micrograms/dose
Do not use BUDESONIDA ALCON 100 micrograms/dose
If you are allergic (hypersensitive) to budesonide or any of the other components of BUDESONIDA ALCON 100 micrograms/dose (listed in section 6).
Warnings and Precautions
Consult your doctor or pharmacist before starting to use BUDESONIDA ALCON 100 micrograms/dose.
- Patient with pulmonary tuberculosis or with fungal or viral infections of the respiratory tract, or those patients previously treated with systemic corticosteroids (oral or injectable), should be treated with special caution and under medical supervision.
- In long-term treatments, a review of the nasal mucosa should be performed at least once a year.
- The use of the spray by more than one person can lead to contagion.
- During treatment with this preparation, it should be taken into account that there is a potential risk of masking a local infection.
- After the treatment period, it is recommended to discard the remaining preparation, even if it has not been fully consumed.
- Contact your doctor if you experience blurred vision or other visual disturbances.
Children
Children should use BUDESONIDA ALCON 100 micrograms/dose only under the supervision of an adult to ensure correct administration and that the dose corresponds to that prescribed by the doctor.
Using BUDESONIDA ALCON 100 micrograms/dose with other medicines
No interactions have been described, although it is recommended to inform your doctor or pharmacist if you are using, or have recently used, any other medicine, including those purchased without a prescription.
It may be useful to administer an antihistamine eye drop simultaneously to counteract the ocular effects produced by the allergy.
Pregnancy and Breastfeeding
It will only be used during pregnancy and breastfeeding when the doctor considers it necessary. Consult your doctor or pharmacist before using any medicine.
Driving and Using MachinesNo effects on the ability to drive and use machines have been described.
This medicine contains potassium sorbate
This medicine may cause local skin reactions (such as contact dermatitis) because it contains potassium sorbate as an excipient.
Athletes are informed that this medicine contains budesonide, which may result in a positive doping test.
3. How to use BUDESONIDA ALCON 100 micrograms/dose
Follow the administration instructions of BUDESONIDA ALCON 100 micrograms/dose exactly as indicated by your doctor. In case of doubt, consult your doctor or pharmacist.
The dosage should be individualized and may be modified according to medical criteria.
In adults and children from 6 years old, the recommended habitual dose is two applications (200 mcg) in each nostril once a day, in the morning (total 400 mcg).
When symptoms start to improve, the dose can be reduced to one application (100 mcg) in each nostril (total 200 mcg).
Several days are required for the symptoms to disappear completely, using the recommended doses and intervals.
Treatment of seasonal rhinitis should be started, whenever possible, before exposure to the allergen.
In the treatment of nasal polyps, the daily dose can be increased up to 800 mcg.
The total daily dose can be divided, administered in the morning and at night, according to medical criteria.
Instructions for the correct administration of the preparation
Before the first application:
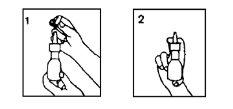 Remove the protective cap.
Remove the protective cap.- Shake the bottle-applicator assembly.
- Actuate the actuator the necessary number of times so that the valve mechanism is filled and a correct spray can be produced.
Mode of Use. For each application:
|
Repeat the same process in the other nostril.
After use, replace the protective cap.
The use of the spray by more than one person can lead to contagion.
If you use more BUDESONIDA ALCON 100 micrograms/dose than you should
Although no toxic symptoms are expected in case of overdose or accidental ingestion, in such cases, consult the Toxicology Information Service. Telephone 91 562 04 20.
If you observe symptoms of edema, facial swelling, or moon face, etc., you should inform your doctor so that the appropriate measures can be taken.
If you forget to use BUDESONIDA ALCON 100 micrograms/dose
Do not use a double dose to make up for forgotten doses.
If you have any other questions about the use of this medicine, ask your doctor or pharmacist.
4. Possible side effects
Like all medicines, BUDESONIDA ALCON 100 micrograms/dose can cause side effects, although not everyone gets them.
Occasionally, sneezing fits may occur immediately after using the spray.
Rarely, a mild nasal hemorrhage, nasal dryness, and throat irritation or blurred vision may occur.
Exceptionally, cases of cutaneous allergic reactions associated with the use of the preparation have been described. Extremely rare cases of nasal septum perforation have been reported after the use of nasal corticosteroids.
If you consider that any of the side effects you are experiencing is serious or if you notice any side effect not mentioned in this package leaflet, consult your doctor or pharmacist.
5. Storage of BUDESONIDA ALCON 100 micrograms/dose
No special storage conditions are required.
Keep this medicine out of the reach and sight of children.
Do not use BUDESONIDA ALCON 100 micrograms/dose after the expiration date stated on the packaging. The expiration date is the last day of the month indicated.
Medicines should not be disposed of via wastewater or household waste. Deposit the packaging and any unused medicines in the SIGRE collection point at the pharmacy. If in doubt, ask your pharmacist how to dispose of the packaging and any unused medicines. This will help protect the environment.
6. Additional Information
Composition of BUDESONIDA ALCON 100 micrograms/dose
- Each 100 ml contains 0.2 g of budesonide (DCI).
- Each dose provides 100 mcg of budesonide. A spray bottle contains 200 doses.
- Excipients: glucose, potassium sorbate, disodium edetate, cellulose, and sodium carboxymethylcellulose, polysorbate 80, and purified water.
Appearance of the Product and Package Contents
BUDESONIDA ALCON 100 micrograms/dose is a medicine in the form of a nasal spray suspension, presented in glass bottles with 10 ml (200 doses of 100 mcg/dose).
Marketing Authorization Holder and Manufacturer
Marketing Authorization Holder
M4 PHARMA, S.L.
C/ Tánger, 86
08018 Barcelona (Spain)
Manufacturer
LABORATORIO REIG JOFRE, S.A.
Gran Capita, 10.
Sant Joan Despi, Barcelona – Spain
This package leaflet was revised in June 2017
Detailed and updated information on this medicine is available on the website of the Spanish Agency for Medicines and Health Products (AEMPS) http://www.aemps.gob.es/
- Country of registration
- Average pharmacy price8.12 EUR
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to BUDESONIDE ALCON 100 micrograms/DOSE NASAL SPRAY SUSPENSIONDosage form: NASAL PRODUCT, 64 MCG/sprayActive substance: budesonideManufacturer: Laboratorio Aldo Union S.L.Prescription requiredDosage form: NASAL PRODUCT, 1 mg budesonide/mlActive substance: budesonideManufacturer: M4 Pharma S.L.Prescription requiredDosage form: NASAL PRODUCT, 64 mcg/per actuationActive substance: budesonideManufacturer: Laboratorio Aldo Union S.L.Prescription required
Online doctors for BUDESONIDE ALCON 100 micrograms/DOSE NASAL SPRAY SUSPENSION
Discuss questions about BUDESONIDE ALCON 100 micrograms/DOSE NASAL SPRAY SUSPENSION, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions











