
RHINOCORT 64 micrograms NASAL SPRAY SUSPENSION

How to use RHINOCORT 64 micrograms NASAL SPRAY SUSPENSION
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the User
Rhinocort 64 micrograms nasal spray suspension
Budesonide
Read all of this leaflet carefully before you start using this medicine because it contains important information for you. Follow exactly the instructions for administration of the medicine contained in this leaflet or as indicated by your doctor or pharmacist.
|
Contents of the Package Leaflet
- What Rhinocort 64 micrograms is and what it is used for.
- What you need to know before you start using Rhinocort 64 micrograms.
- How to use Rhinocort 64 micrograms.
- Possible side effects.
- Storage of Rhinocort 64 micrograms.
- Contents of the pack and further information.
1. What Rhinocort 64 micrograms is and what it is used for
Rhinocort 64 micrograms contains the active ingredient budesonide, which belongs to a group of medicines called glucocorticoids, and is used to reduce inflammation. Rhinocort 64 micrograms reduces the inflammation of the nasal mucosa (the inside of the nose).
Rhinocort 64 micrograms is used for the relief of nasal symptoms (such as sneezing, itching, runny nose, congestion) of allergic rhinitis caused by pollen and other allergens present in the air, such as dust mites, fungal spores, or animal hair, in adults.
The maximum level of protection may not be achieved during the first few days, so it is essential to continue regular use to ensure complete therapeutic benefit.
You should consult a doctor if your symptoms do not improve or worsen after 7 days.
This medicine should not be used continuously for more than 3 months, unless your doctor advises you to do so.
2. What you need to know before you start using Rhinocort 64 micrograms
Do not use Rhinocort 64 micrograms:
- If you are allergic to the active ingredient or any of the other ingredients of this medicine (listed in section 6).
- If you have symptoms or signs of a localized infection in the nasal passages.
Warnings and precautions
Consult your doctor or pharmacist before starting to use Rhinocort 64 micrograms if:
- You suffer from severe or frequent nosebleeds
- You have had recent nasal ulcers, have undergone nasal surgery, or have had a nasal injurythat has not healed well.
- You suffer from tuberculosis, chest infection, chickenpox, or measles, or have been in contact with someone who has tuberculosis, chickenpox, or measles.
- You have ever had glaucoma(increased eye pressure) or cataracts.
- You have an eye infection.
- You have diabetes.
- You have liver problems.
Consult your doctor or pharmacist if you experience any of the following while using Rhinocort 64 micrograms:
- Blurred visionor other visual disturbances
- Any sign of infection, such as persistent fever
If your symptoms persist or worsen, or if you experience new symptoms, you should stop using Rhinocort 64 micrograms and consult your doctor.
Children and adolescents
Rhinocort 64 micrograms is not intended for use in children and adolescents under 18 years of age.
Using Rhinocort 64 micrograms with other medicines:
Tell your doctor or pharmacist if you are using, have recently used, or might use any other medicines. This includes medicines obtained without a prescription and herbal remedies. Some medicines may increase the effects of Rhinocort. In particular, tell your doctor or pharmacist if you are taking the following medicines:
- For treating fungal infections(such as itraconazole or ketoconazole).
- You are taking antibiotics(such as clarithromycin)
- You are taking HIV medicines(such as saquinavir, atazanavir, indinavir, nelfinavir, ritonavir, or medicines containing cobicistat).
- You are taking cimetidine(a medicine for stomach acidity).
- You are taking estrogensas hormone replacement therapyor birth control pills.
- You are taking another corticosteroid medicine(such as creams for eczema, inhalers for asthma, tablets, injections, nasal sprays, eye drops, or nose drops).
- You have recently stopped using corticosteroid tabletssuch as prednisoloneor corticosteroid injections.
Pregnancy and breastfeeding
If you are pregnant or breastfeeding, think you may be pregnant, or plan to become pregnant, consult your doctor or pharmacist before using this medicine. You should contact your doctor as soon as possible if you become pregnant during treatment with Rhinocort 64 micrograms.
Driving and using machines
There is no indication that Rhinocort 64 micrograms may affect your ability to drive or operate machinery.
Rhinocort 64 micrograms contains potassium sorbate.
Since Rhinocort 64 micrograms contains potassium sorbate as an excipient, it may cause local skin reactions (e.g., contact dermatitis)
Warning to athletes:Athletes are informed that this medicine contains a component that may produce a positive result in doping tests.
3. How to use Rhinocort 64 micrograms
Follow exactly the instructions for administration of Rhinocort 64 micrograms contained in this leaflet or as indicated by your doctor or pharmacist. If you are in doubt, consult your doctor or pharmacist again.
Adults:
The recommended initial daily dose is two sprays in each nostril.
This dose can be administered as two applications (2 x 64 micrograms) in each nostril once a day in the morning or one application (1 x 64 micrograms) in each nostril, in the morning and in the evening.
Once complete relief of symptoms is achieved, the administration can be reduced to one application in each nostril once a day, preferably in the morning.
Do not administer more than four applications in a day.
You should consult a doctor or pharmacist if your symptoms worsen or do not improve after 7 days.
Do not use this medicine continuously for more than 3 months.
You may notice relief of symptoms from the first day of treatment; however, it may take several days of treatment for the full effect to occur (sometimes up to 2 weeks).
Do not share the spray with other people due to the risk of infection.
Avoid contact of the product with the eyes. If contact with the eyes occurs, rinse them immediately with plenty of water.
Before using Rhinocort 64 micrograms for the first time, you should read the section “Method of administration”, which explains how the spray pump works. Follow the instructions carefully.
Use in children
This medicine should not be used in children or adolescents under 18 years of age.
If you use more Rhinocort 64 micrograms than you should
Administration of more than the recommended dose on a single occasion is unlikely to cause harmful effects. If this occurs over a long period (months), it is possible that side effects may appear, so you should consult your doctor or pharmacist.
In case of overdose or accidental ingestion, go immediately to a medical center or call the Toxicology Information Service, phone +34915620420, indicating the medicine and the amount ingested.
If you forget to use Rhinocort 64 micrograms:
If you forget a dose of Rhinocort 64 micrograms, wait for the next one. Do not take a double dose to make up for the forgotten doses. Simply apply the next dose as needed.
Method of administration
Read the following instructions carefully and follow them.
Before using the Rhinocort 64 micrograms nasal spray for the first time, shake the container, remove the protective cap, and, keeping the container in a vertical position, press several times (5-10 times) into the air until a uniform spray of the product appears (see Figure 1). If you do not use it daily, you need to recharge the pump. In this case, it will be enough to perform a single (1) spray into the air.
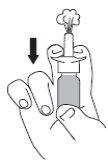
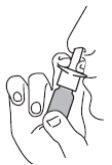
- Blow your nose. Shake the container and remove the cap.
- Hold the container in a vertical position as shown in the figures.
- Insert the tip of the applicator into a nostril and direct it to the side of the nose in the direction opposite the center of the nose (the “septum”). Spray one or two times, depending on the dose needed. Repeat the action in the other nostril.
- Replace the cap. Do not use Rhinocort 64 micrograms nasal spray more times than recommended in this leaflet.
Cleaning
Regularly clean the upper plastic parts. To do this, remove the cap and the nasal applicator. Wash the plastic parts with warm water. Let them dry completely in the air before putting them back.
Do not attempt to clean the nasal applicator with a pin or any sharp object.
When you no longer need this medicine, it is recommended to dispose of the remainder, even if it has not been fully consumed.
If you have any further questions about the use of this medicine, ask your doctor or pharmacist.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
If you experience any of the following, stop using this nasal spray and consult a doctor immediately:
- Sudden signs of allergic reaction such as rash, itching, hives, or redness of the skin, swelling of the face, lips, mouth, tongue, or other parts of the body, difficulty breathing, wheezing, or difficulty swallowing or breathing or feeling of fainting.
Other side effects include:
Frequent (may affect 1 in 10 patients):
- Nasal, throat, or sinus infections:
- Nosebleeds (epistaxis) or nasal irritation.
- Pain in the mouth and/or throat.
- Ear infections
- Headache
- Abdominal discomfort
- Fever
Uncommon (may affect up to 1 in 100 patients):
- Muscle cramps.
Rare (may affect up to 1 in 1,000 patients):
- Effects on the adrenal glands (small glands located next to the kidneys).
- Nasal ulcers or perforation of the membrane that separates the nostrils (nasal septum).
- Voice changes
- Bruises
- Blurred vision.
Other side effects of unknown frequency include:
- Cataracts (loss of transparency of the lens in the eye).
- Glaucoma (increased eye pressure).
- Angioedema.
- Dermatitis.
- Erythema.
- Rash.
- Urticaria.
Other side effects in children
Slower growth has been reported in children treated with nasal corticosteroids.
In children, the following side effects have also been frequently reported: stomach upset, headache, cough, fever or high temperature, inflammation, and infections of the ears, tonsils, sinuses, or lungs, or skin rash.
Mental or behavioral disorders, such as hyperactivity, sleep disturbances, nervousness, depression, or aggression, have been reported rarely (especially in children).
Reporting of side effects
If you experience any side effects, consult your doctor or pharmacist, even if they are not listed in this leaflet. You can also report them directly through the Spanish Medicines Monitoring System for Human Use: www.notificaRAM.es. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of Rhinocort 64 micrograms
Replace the protective cap after using Rhinocort 64 micrograms.
Do not store above 30 °C. Do not freeze.
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date stated on the container after “EXP”. The expiry date is the last day of the month indicated.
Medicines should not be disposed of via wastewater or household waste. Dispose of the container and any unused medicine in the pharmacy’s SIGRE collection point. Ask your pharmacist how to dispose of the container and any unused medicine. This will help protect the environment.
6. Contents of the pack and further information
Composition of Rhinocort 64 micrograms
- The active ingredient is budesonide.
- The other ingredients (excipients) are: disodium edetate, potassium sorbate (E 202), anhydrous glucose, microcrystalline cellulose (E 460), and sodium carboxymethyl cellulose (E 466), polysorbate 80 (E 433), hydrochloric acid, and purified water.
Appearance and packaging of the product
Rhinocort 64 micrograms is presented in a 10 ml brown glass bottle (120 sprays) with a spray pump and a nasal applicator. The liquid it contains is a suspension intended for nasal spraying. Each spray contains 64 micrograms of the active ingredient.
Marketing authorization holder and manufacturer
The marketing authorization holder is:
JNTL Consumer Health (Spain), S.L.
C/ Vía de los Poblados 1, Edificio E, planta 3
28033-Madrid
Spain
The manufacturer is:
McNeil AB
Norrbroplatsen 2
251 09 Helsingborg
Sweden
Date of last revision of this leaflet: March 2022
Detailed information about this medicine is available on the website of the Spanish Agency for Medicines and Health Products (AEMPS) (http://www.aemps.gob.es/)
- Country of registration
- Active substance
- Prescription requiredNo
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to RHINOCORT 64 micrograms NASAL SPRAY SUSPENSIONDosage form: NASAL PRODUCT, 64 MCG/sprayActive substance: budesonideManufacturer: Laboratorio Aldo Union S.L.Prescription requiredDosage form: NASAL PRODUCT, 2 mg budesonide/ mlActive substance: budesonideManufacturer: M4 Pharma S.L.Prescription requiredDosage form: NASAL PRODUCT, 1 mg budesonide/mlActive substance: budesonideManufacturer: M4 Pharma S.L.Prescription required
Online doctors for RHINOCORT 64 micrograms NASAL SPRAY SUSPENSION
Discuss questions about RHINOCORT 64 micrograms NASAL SPRAY SUSPENSION, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions











