
How to use Ovamex
PATIENT INFORMATION LEAFLET
Leaflet accompanying the packaging: patient information
Ovamex
0.25 mg/0.5 ml, solution for injection in a pre-filled syringe
Ganirelix
Read the leaflet carefully before taking the medicine, as it contains important information for the patient.
- Keep this leaflet, so you can read it again if you need to.
- If you have any doubts, consult your doctor, pharmacist, or nurse.
- This medicine has been prescribed specifically for you. Do not pass it on to others. The medicine may harm another person, even if their symptoms are the same.
- If the patient experiences any side effects, including any not listed in this leaflet, they should tell their doctor or pharmacist. See section 4.
Table of contents of the leaflet
- 1. What is Ovamex and what is it used for
- 2. Important information before taking Ovamex
- 3. How to take Ovamex
- 4. Possible side effects
- 5. How to store Ovamex
- 6. Contents of the pack and other information
1. What is Ovamex and what is it used for
Ovamex contains the active substance ganirelix and belongs to a group of medicines called "gonadotropin-releasing hormone antagonists". Gonadotropin-releasing hormone (GnRH) regulates the release of gonadotropins [luteinizing hormone (LH) and follicle-stimulating hormone (FSH)]. Gonadotropins play an essential role in human fertility and reproduction. In women, FSH is necessary for the growth and development of follicles in the ovaries. The follicles contain egg cells. LH is necessary for the release of mature egg cells from the follicles and ovaries (i.e., ovulation). Ovamex inhibits the action of GnRH, which leads to the inhibition of hormone release, primarily LH. Ovamex is used in women participating in assisted reproduction programs, including in vitro fertilization (IVF) and other methods. Occasionally, ovulation may occur too early, reducing the chances of pregnancy. Ovamex is used to prevent premature LH surge, which may cause too early release of egg cells.
2. Important information before taking Ovamex
When not to take Ovamex
- if the patient is allergic to ganirelix or any of the other ingredients of this medicine (listed in section 6);
- if the patient has been diagnosed with an allergy to gonadotropin-releasing hormone (GnRH) or a GnRH analogue;
- if the patient has moderate or severe kidney or liver disease;
- if the patient is pregnant or breastfeeding.
Warnings and precautions
Before starting treatment with Ovamex, discuss it with your doctor, pharmacist, or nurse.
- if the patient has an active allergic condition, they should inform their doctor. The doctor will decide, depending on the severity of the condition, whether additional monitoring is necessary during treatment. Allergic reactions have been reported after the first dose of the medicine;
- both generalized and localized allergic reactions have been reported, including hives, facial swelling, lip, tongue, and/or throat swelling, which may cause difficulty breathing and/or swallowing (angioedema and/or anaphylaxis) (see also section 4). If an allergic reaction occurs, the patient should stop taking Ovamex and seek medical help immediately;
- during hormonal stimulation of the ovaries or after it, ovarian hyperstimulation syndrome (OHSS) may occur. This syndrome is associated with gonadotropin stimulation procedures. The patient should read the patient information leaflet accompanying the prescribed gonadotropin-containing medicines;
- the frequency of congenital malformations after assisted reproduction techniques may be slightly higher than after natural conception. This is likely related to other characteristics of patients undergoing fertility treatment (e.g., the woman's age, semen parameters) and the more frequent occurrence of multiple pregnancies after assisted reproduction techniques. The frequency of congenital malformations after assisted reproduction techniques using Ovamex does not differ from the frequency of congenital malformations after using other GnRH analogues during assisted reproduction techniques;
- women with damaged fallopian tubes have a slightly increased risk of ectopic pregnancy;
- The efficacy and safety of Ovamex in women with a body weight below 50 kg or above 90 kg have not been established. For further information, the patient should consult their doctor.
Children and adolescents
Ovamex should not be used in children and adolescents.
Ovamex and other medicines
The patient should tell their doctor or pharmacist about all medicines they are taking or have recently taken, as well as any medicines they plan to take.
Pregnancy, breastfeeding, and fertility
If the patient is pregnant or breastfeeding, thinks they may be pregnant, or plans to have a baby, they should consult their doctor or pharmacist before taking this medicine. Ovamex should be used during controlled ovarian stimulation as part of assisted reproduction techniques (ART). Do not take Ovamex during pregnancy and breastfeeding.
Driving and using machines
The effect of Ovamex on the ability to drive and use machines has not been studied.
Ovamex contains sodium
The medicine contains less than 1 mmol of sodium (23 mg) per injection, which means it is considered "sodium-free".
3. How to take Ovamex
This medicine is used as part of assisted reproduction programs (ART), including in vitro fertilization (IVF). The injections will be administered by the patient themselves, so the doctor will explain what to do. The medicine should always be used according to the doctor's or pharmacist's instructions. If the patient does not understand the instructions, they should consult their doctor or pharmacist.
Step 1
Ovarian stimulation with follicle-stimulating hormone (FSH) or follitropin alpha may start on the 2nd or 3rd day of menstrual bleeding.
Step 2
The contents of the Ovamex syringe (0.25 mg) should be injected subcutaneously once a day, starting from the 5th or 6th day of stimulation. Depending on the ovarian response, the doctor may decide to start treatment on a different day.
- The treatment with Ovamex and FSH should be started approximately at the same time. However, the medicines should not be mixed, and the injections should be administered at different sites.
Ovamex and FSH should be administered until the day when the follicles have reached the appropriate size.
Step 3
Final maturation of the egg cells in the follicles can be induced by administering human chorionic gonadotropin (hCG). The time between the two injections of Ovamex and the time between the last injection of Ovamex and the injection of hCG should not exceed 30 hours, as premature ovulation (i.e., release of egg cells) may occur. Therefore, if Ovamex was injected in the morning, Ovamex should also be taken on the day when hCG is administered to induce ovulation. If Ovamex was injected in the afternoon, the last injection of Ovamex should be administered in the afternoon before the day of ovulation induction.
Instructions for use
- Injection siteOvamex is provided in pre-filled syringes containing a single dose. The contents should be injected slowly, directly under the skin, preferably in the thigh. The solution should be checked before administration. Do not use if the solution contains particles or is not clear. Air bubbles may be visible in the syringe. This is normal, and removing the air bubble is not necessary. When self-injecting or injecting with the help of a partner, follow the instructions below carefully. Do not mix the medicinal product Ovamex with other medicines.
- Preparing the injection siteWash your hands with soap and water. Clean the injection site with a disinfectant (e.g., alcohol) to remove bacteria from the skin surface. Clean an area of about 5 cm (2 inches) around the injection site and let the disinfectant dry for at least one minute before administering the injection.
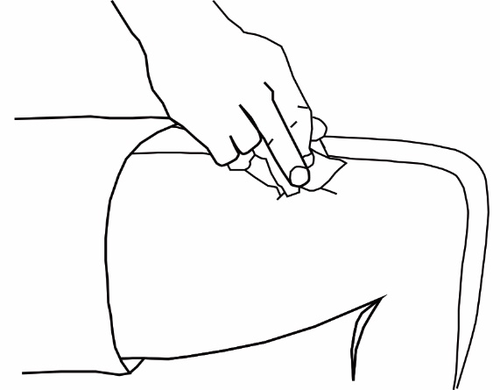
- Inserting the needleRemove the needle cover. Gently grasp the clean skin, creating a fold. Insert the needle at the base of the grasped skin. Insert the needle at a 45° angle to the skin surface.
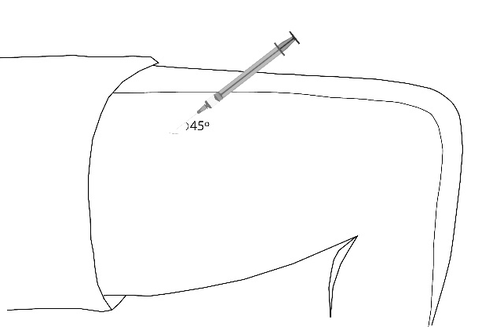
Change the injection site each time.
- Checking the correct position of the needleGently pull back the syringe plunger to check if the needle is correctly positioned. If blood is drawn into the syringe, it means the needle tip has punctured a blood vessel. Do not continue injecting Ovamex. Remove the syringe, cover the injection site with a swab soaked in a disinfectant, and press; the bleeding should stop within about a minute. Do not reuse the syringe and dispose of it properly. Try again with a new syringe.
- Injecting the solutionWhen the needle is correctly positioned, slowly and evenly press the syringe plunger so that the solution is injected properly without damaging the skin tissue.
- Removing the syringeQuickly remove the syringe. Cover the injection site with a swab soaked in a disinfectant. Use the pre-filled syringe only once.
Using more Ovamex than recommended
Contact your doctor.
Missing a dose of Ovamex
If the patient remembers missing a dose, they should take it as soon as possible. Do not take a double dose to make up for the missed dose. If the delay is more than 6 hours (so that the time between two injections is longer than 30 hours), take the dose as soon as possible and consult your doctor for advice.
Stopping Ovamex treatment
Do not stop taking Ovamex unless your doctor advises you to, as this may affect the treatment results. If you have any further doubts about using this medicine, consult your doctor or pharmacist.
4. Possible side effects
Like all medicines, Ovamex can cause side effects, although not everybody gets them.
Very common (may affect more than 1 in 10 patients)
- Local skin reactions at the injection site (mainly redness, with or without swelling). The local reaction usually disappears within 4 hours after administration.
Uncommon (may affect up to 1 in 100 patients)
- headache
- nausea
- malaise.
Rare (may affect up to 1 in 10,000 patients)
- Allergic reactions have been reported after the first dose.
- rash
- facial swelling
- breathing difficulties (dyspnea)
- facial, lip, tongue, and/or throat swelling, which may cause difficulty breathing and/or swallowing (angioedema and/or anaphylaxis)
- hives.
Additionally, side effects have been reported that are known to occur during controlled ovarian hyperstimulation, such as:
- abdominal pain;
- ovarian hyperstimulation syndrome (OHSS). (OHSS occurs when the ovaries overreact to fertility medicines.)
- ectopic pregnancy (when the embryo develops outside the uterus)
- miscarriage (see the patient information leaflet of the prescribed FSH-containing medicine).
In one patient, exacerbation of a pre-existing rash was reported after the first dose of ganirelix.
Reporting side effects
If the patient experiences any side effects, including any not listed in this leaflet, they should tell their doctor or pharmacist. Side effects can be reported directly to the Department of Drug Safety, Office for Registration of Medicinal Products, Medical Devices, and Biocidal Products, Al. Jerozolimskie 181C, 02-222 Warsaw, Tel.: +48 22 49 21 301, Fax: +48 22 49 21 309. Website: https://smz.ezdrowie.gov.pl. Side effects can also be reported to the marketing authorization holder. By reporting side effects, you can help provide more information on the safety of this medicine.
5. How to store Ovamex
Keep the medicine out of the sight and reach of children. Do not use this medicine after the expiry date stated on the packaging after "EXP". The expiry date refers to the last day of the month. There are no special precautions for storage. Check the pre-filled syringe before use. Only use pre-filled syringes containing a clear solution without particles and with undamaged packaging. Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. This will help protect the environment.
6. Contents of the pack and other information
What Ovamex contains
- The active substance is ganirelix. Each pre-filled syringe contains 0.25 mg of ganirelix (as acetate) in 0.5 ml of aqueous solution.
- The other ingredients are acetic acid, mannitol, and water for injections. The appropriate pH (acidity) was adjusted using sodium hydroxide and acetic acid.
What Ovamex looks like and contents of the pack
Ovamex is a clear and colorless aqueous solution for injection. The solution is ready for use and intended for subcutaneous administration. Ovamex is available in packs containing 1 or 5 pre-filled syringes with injection needles (27 G), as specified below:
- 1 pre-filled syringe
- 5 pre-filled syringes
Not all pack sizes may be marketed.
Marketing authorization holder
Theramex Ireland Limited
3 Floor, Kilmore House,
Park Lane, Spencer Dock,
Dublin 1
D01 YE64
Ireland
Manufacturer
GP-PHARM, S.A.
Polígono Industrial Els Vinyets-Els Fogars, Sector 2,
Carretera Comarcal C-244, Km 22,
08777 Sant Quintí de Mediona, Spain
This medicinal product is authorized in the Member States of the European Economic Area under the following names:
Portugal:
Belgium:
Poland:
Greece:
Czech Republic:
Italy:
Information about this medicine can be obtained by calling +48 22 307 71 66
Date of last revision of the leaflet:
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- ImporterGP-PHARM S.A.
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to OvamexDosage form: Solution, 60 mgActive substance: lanreotidePrescription requiredDosage form: Solution, 90 mgActive substance: lanreotidePrescription required
Alternatives to Ovamex in other countries
The best alternatives with the same active ingredient and therapeutic effect.
Alternative to Ovamex in Ukraine
Alternative to Ovamex in Spain
Online doctors for Ovamex
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for Ovamex – subject to medical assessment and local rules.







