
How to use Lanreotide Zentiva
Leaflet accompanying the packaging: information for the user
Lanreotide Zentiva, 60 mg, solution for injection in a pre-filled syringe
Lanreotide Zentiva, 90 mg, solution for injection in a pre-filled syringe
Lanreotide Zentiva, 120 mg, solution for injection in a pre-filled syringe
Lanreotide
Read the leaflet carefully before using the medicine, as it contains important information for the patient.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their symptoms are the same as yours.
- If you experience any side effects, including those not listed in this leaflet, please tell your doctor or pharmacist. See section 4.
Table of contents of the leaflet
- 1. What is Lanreotide Zentiva and what is it used for
- 2. Important information before using Lanreotide Zentiva
- 3. How to use Lanreotide Zentiva
- 4. Possible side effects
- 5. How to store Lanreotide Zentiva
- 6. Contents of the pack and other information
1. What is Lanreotide Zentiva and what is it used for
What is Lanreotide Zentiva and how does it work
This medicine is called Lanreotide Zentiva.
The active substance of this medicine provides long-lasting action.
Lanreotide - the active substance - belongs to a group of medicines called growth hormone inhibitors. It
is similar to another substance (hormone) called somatostatin.
Lanreotide causes a decrease in the activity of hormones such as growth hormone (GH) and insulin-like growth factor 1 (IGF-1), as well as inhibiting the release of certain gastrointestinal hormones and intestinal secretion. Additionally, it also has an effect on certain advanced types of tumors (called neuroendocrine tumors) occurring in the intestines and pancreas, by inhibiting or delaying their growth.
What is Lanreotide Zentiva used for:
- in the long-term treatment of patients with acromegaly (a condition in which the body produces too much growth hormone)
- to alleviate symptoms associated with acromegaly - such as fatigue, headaches, sweating, joint pain, numbness of hands and feet
- to alleviate symptoms such as hot flashes and diarrhea, which sometimes occur in patients with neuroendocrine tumors (NETs)
- to treat and inhibit the growth of certain advanced tumors occurring in the intestines and pancreas, called gastroenteropancreatic neuroendocrine tumors (GEP-NETs). It is used when these tumors cannot be surgically removed.
2. Important information before using Lanreotide Zentiva
When not to use Lanreotide Zentiva
- if the patient is allergic (hypersensitive) to lanreotide, somatostatin or medicines belonging to the same group (somatostatin analogs) or any of the other ingredients of this medicine (listed in section 6).
Warnings and precautions
Before starting treatment with Lanreotide Zentiva, discuss with your doctor or pharmacist:
- if the patient has diabetes, as lanreotide can cause fluctuations in blood sugar levels. During treatment with lanreotide, the doctor may recommend checking blood sugar levels and possibly changing the diabetes treatment regimen.
- if the patient has been diagnosed with gallstones, as lanreotide can contribute to the formation of gallstones. In this case, periodic examination is recommended. The doctor may decide to discontinue treatment with lanreotide if complications arise from gallstone formation.
- if the patient has any thyroid function disorders, as lanreotide may slightly disrupt the function of this organ.
- if the patient has heart function disorders, as lanreotide may cause sinus bradycardia (slow heart rate). In patients with bradycardia, lanreotide should be used with particular caution.
If any of the above points apply to the patient, before taking Lanreotide Zentiva, discuss it with your doctor or pharmacist.
Consult a doctor or pharmacist if, during treatment:
- The patient experiences fatty stools, loose stools, abdominal bloating or weight loss, as lanreotide may affect the secretion of pancreatic enzymes involved in food digestion.
Children and adolescents
Lanreotide Zentiva is not recommended for children and adolescents.
Lanreotide Zentiva and other medicines
Some medicines can affect the action of other medicines. Tell your doctor or pharmacist about all medicines you are currently taking or have recently taken, as well as any medicines you plan to take.
Particular caution should be exercised when using concomitantly with:
- Cyclosporine(a medicine that reduces the immune system response, used after transplantation or in the case of autoimmune disease),
- Bromocriptine(a dopamine receptor agonist, used to treat certain types of brain tumors and Parkinson's disease or to inhibit lactation after childbirth),
- Antidiabetic medicines(medicines that lower high blood glucose levels),
- Medicines that cause bradycardia(medicines that slow down heart rate, e.g. beta-blockers).
The doctor may consider modifying the dosage of the above medicines used concomitantly.
Pregnancy and breastfeeding
If the patient is pregnant or breastfeeding, thinks she may be pregnant or plans to have a child, she should consult a doctor or pharmacist before using this medicine. Lanreotide Zentiva should only be administered if clearly necessary.
Driving and using machines
It is unlikely that Lanreotide Zentiva will affect the ability to drive or use machines, but during treatment with Lanreotide Zentiva, side effects such as dizziness may occur. If such a side effect occurs in the patient, they should not drive vehicles or operate machines.
3. How to use Lanreotide Zentiva
Lanreotide Zentiva should always be used as directed by the doctor. In case of doubts, consult a doctor or pharmacist.
Recommended dose
Treatment of acromegaly
The recommended dose is one injection every 28 days. The dose of the medicine used for injection will be selected by the doctor from the three available strengths of Lanreotide Zentiva (60 mg, 90 mg or 120 mg).
If a satisfactory response is achieved, the doctor may recommend changing the frequency of injections of Lanreotide Zentiva 120 mg to one injection every 42 or 56 days.
The doctor will also decide on the duration of treatment.
Relief of symptoms (such as hot flashes and diarrhea) associated with neuroendocrine tumors
The recommended dose is one injection of the medicine every 28 days. The dose of the medicine used for injection will be selected by the doctor from the three available strengths of Lanreotide Zentiva (60 mg, 90 mg or 120 mg).
If a satisfactory response is achieved to treatment with a somatostatin analog or Lanreotide Zentiva 60 mg or 90 mg, the doctor may recommend changing the frequency of injections of Lanreotide Zentiva 120 mg to one injection every 42 or 56 days.
The doctor will also decide on the duration of treatment.
Treatment of advanced tumors occurring in the intestines and pancreas, called gastroenteropancreatic neuroendocrine tumors (GEP-NETs). The medicine is used when these tumors cannot be surgically removed.
The recommended dose is 120 mg every 28 days. The doctor will decide on the duration of treatment with Lanreotide Zentiva to inhibit tumor growth.
Method of administration
Lanreotide Zentiva should be administered by deep subcutaneous injection.
The injection should be performed by a healthcare professional or a trained person (family member or friend) or by the patient themselves after proper training by a healthcare professional.
The decision to self-administer or administer by another trained person should be made by the doctor. If the patient has any doubts about the method of injection, they should consult a doctor or healthcare professional for advice or further training.
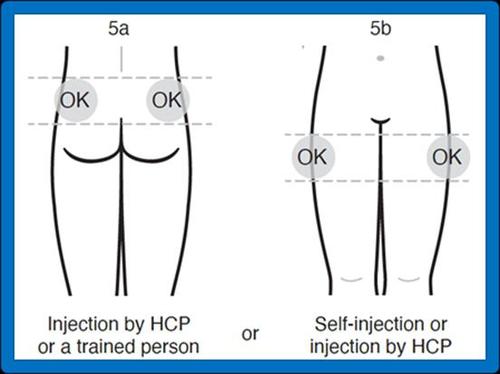
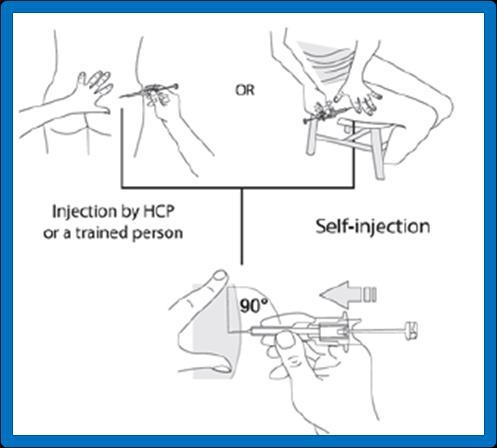
If the injection is performed by a healthcare professional or a trained person (family member or friend), the injection should be given in the upper outer quadrant of the buttock or in the upper outer part of the thigh (see figures 5a and 5b below).
If the patient is self-administering the injection after proper training, the injection should be given in the upper outer part of the thigh (see figure 5b below).
Instructions for use
Important: Read the entire instructions before performing the injection. Deep subcutaneous injection requires a special technique, different from that used for standard subcutaneous injection.
The following instructions explain how to perform the injection of Lanreotide Zentiva.
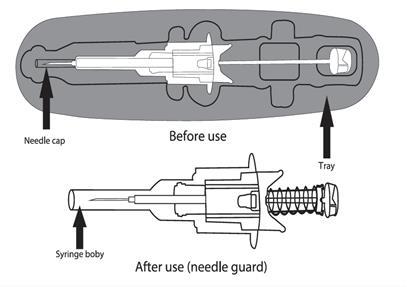
Lanreotide Zentiva is provided in a ready-to-use pre-filled syringe with an automatic safety system. The needle will retract automatically after completing the full injection of the contents to prevent patient injury.
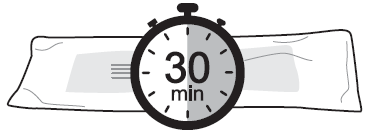
- 1. Remove Lanreotide Zentiva
from the refrigerator 30 minutes before administration.
Injecting cold
contents may be
painful. Store the
laminated pouch
sealed until the moment
of injection.
- 2. Warning: Before opening the pouch, check if it is intact and if the expiry date of the medicine has not been exceeded.
Do not use the pre-filled syringe:
- If it has been damaged or if the pre-filled syringe or its packaging appears to be damaged in any way.
- If the expiry date of the medicine has been exceeded; the expiry date is printed on the pouch and carton.
If any of the above situations occur, contact a doctor or pharmacist.
- 3. Wash your hands with soap.
- 4. Open the pouch along the perforated line and remove the pre-filled syringe. The contents of the pre-filled syringe are a semi-solid substance resembling a thick gel of white to light yellow color. The saturated solution may also contain microbubbles, which may disappear during injection. These differences are normal and do not affect the quality of the product.
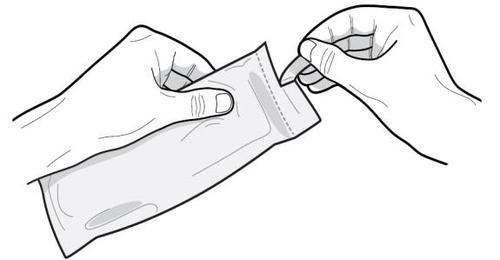
After opening the laminated protective pouch, the medicine should be administered immediately.
- 5. Choose the injection site:
If the injection is performed by a healthcare professional or another trained person (family member or friend):
upper outer quadrant of the buttock (5a) or upper outer part of the thigh (5b).
If the patient is self-administering the injection: upper outer part of the thigh (5b).
- The injection siteof Lanreotide Zentiva should be changedwith each administration, injecting alternately on one side and then the other. Avoid areas where there are moles, scars, redness, or skin irregularities.
- 6. Clean the injection site.
- 7. Before injection, remove the pre-filled syringe from the tray. Discard the tray.
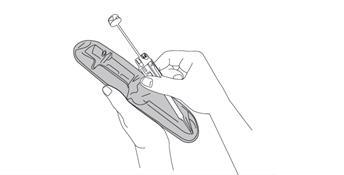
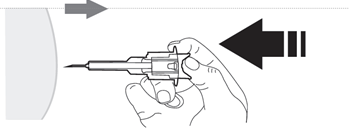
- 8. Pulling, remove the needle cap and discard it.
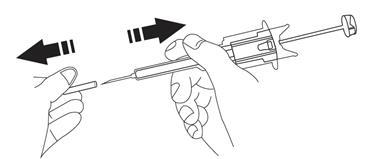
- 9. Flattenthe skin at the injection site using your thumb and index finger of the hand not holding the pre-filled syringe, to stretch it. Do not create a foldin the skin. With a firm, smooth motion, quickly insertthe entire needle perpendicularlyto the skin surface (at a 90-degree angle). It is important that the entire needle is inserted into the body. After inserting the needle, no part of it should be visible.
Do notwithdrawthe needle.
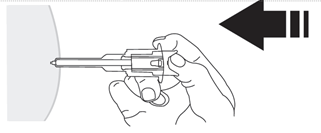
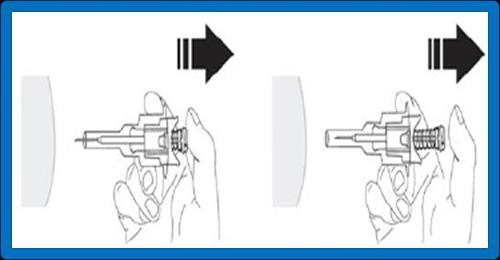
- 10. Release the injection site, which was flattened by your hand. Press the plunger with a smooth, firm motion. The medicine is thicker and more difficult to administer than expected. The injection of the contents usually takes 20 seconds. Make sure to inject the full dose and push the plungerto ensure that no medicine remains in the packaging.

WARNING: Do not release the pressure on the plunger to prevent activation of the automatic safety system.
|  |
| |
 | |
| |
Using a higher dose of Lanreotide Zentiva than recommended
If a higher dose of Lanreotide Zentiva than recommended is administered, inform a doctor.
If a higher dose of Lanreotide Zentiva than recommended is administered, there is a risk of additional or more severe side effects (see section 4. Possible side effects).
Missing a dose of Lanreotide Zentiva
If the patient realizes they have missed an injection, they should contact their doctor, who will provide information on the next injection date.
Do not self-administer additional injections to make up for a missed dose without discussing it with a doctor.
Stopping treatment with Lanreotide Zentiva
Missing more than one dose or stopping treatment with Lanreotide Zentiva prematurely may affect the effectiveness of therapy. Consult a doctor before stopping treatment with the medicine.
If you have any further doubts about using this medicine, consult a doctor or pharmacist.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
If the patient experiences any of the following side effects, they should contact a doctor immediately:
- increased thirst or fatigue, and dry mouth - this may indicate that the patient has high blood sugar levels or is developing diabetes.
- hunger, trembling, excessive sweating, or confusion - these may be symptoms of low blood sugar levels.
These side effects are common, they may occur in up to 1 in 10 people.
Immediately contact a doctor if the patient notices:
- redness or swelling of the face, rash, or hives
- chest tightness, shortness of breath, or wheezing
- fainting, which may be due to a drop in blood pressure.
These may be symptoms of an allergic reaction.
The frequency of this side effect is unknown; it cannot be estimated from the available data.
Other side effects
If the patient experiences any of the following side effects, they should contact a doctor or pharmacist immediately.
The most common side effects expected with Lanreotide Zentiva include gastrointestinal disorders, gallbladder disorders, and injection site reactions. The following side effects are related to the use of Lanreotide Zentiva, including their frequency.
- diarrhea, loose stools, abdominal pain
- gallstones and other gallbladder disorders. Severe and sudden abdominal pain, high fever, jaundice (yellowing of the skin and eyes), chills, loss of appetite, and itching of the skin may occur.
Very common (may occur in more than 1 in 10 people):
- weight loss
- lack of energy
- slow heart rate
- feeling very tired
- decreased appetite
- general weakness
- excess fat in the stool
- dizziness, headache
- hair loss or reduced body hair
- muscle, tendon, or bone pain
- injection site reactions such as pain, skin thickening, or itching
- abnormal liver and pancreas function test results and changes in blood sugar levels
- nausea, vomiting, constipation, gas, bloating, or abdominal discomfort, indigestion
- enlargement of the bile ducts (enlargement of the bile ducts between the liver and the gallbladder and intestine). Abdominal pain, nausea, jaundice, and fever may occur.
Uncommon (may occur in up to 1 in 100 people):
- hot flashes
- difficulty sleeping
- change in stool color
- changes in sodium and alkaline phosphatase levels shown in blood tests.
Frequency not known: frequency cannot be estimated from the available data
- sudden, severe abdominal pain - may be a symptom of pancreatitis
- injection site redness, pain, warmth, swelling, which may feel fluid-filled when pressed, fever - may be symptoms of an abscess
- sudden, severe pain in the upper right or middle abdomen, radiating to the arm or back, abdominal tenderness, nausea, vomiting, and high fever - may be symptoms of cholecystitis
- abdominal pain, fever, chills, jaundice (yellowing of the skin and eyes), nausea, vomiting, clay-colored stools, dark urine, fatigue - may be symptoms of bile duct inflammation
- decreased activity of pancreatic enzymes. Since lanreotide may affect the secretion of pancreatic enzymes involved in food digestion, the patient may experience symptoms such as fatty stools, loose stools, abdominal bloating, or weight loss.
Because Lanreotide Zentiva may cause fluctuations in blood sugar levels, the doctor may recommend regular blood sugar checks, especially at the beginning of treatment.
Similarly, due to the possibility of gallbladder disorders during treatment with this type of medicine, the doctor may recommend regular gallbladder checks at the beginning of Lanreotide Zentiva treatment and at certain time intervals.
If any of the above side effects occur, inform a doctor or pharmacist.
Reporting side effects
If side effects occur, including those not listed in this leaflet, inform a doctor or pharmacist. Side effects can be reported directly to the Department of Adverse Reaction Monitoring of Medicinal Products, Medical Devices, and Biocidal Products,
Jerozolimskie Avenue 181C,
02-222 Warsaw,
phone: +48 22 49 21 301,
fax: +48 22 49 21 309,
website: https://smz.ezdrowie.gov.pl
Side effects can also be reported to the marketing authorization holder or its representative in Poland.
Reporting side effects will help gather more information on the safety of this medicine.
5. How to store Lanreotide Zentiva
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date stated on the carton and label after "EXP". The expiry date refers to the last day of the month stated.
Lanreotide Zentiva should be stored in a refrigerator at a temperature of 2°C - 8°C in the original packaging to protect from light.
After removal from the refrigerator, the product can be stored in a sealed pouch at a temperature below 30°C for a maximum of 72 hours for further storage and later use.
Each syringe is packaged separately.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. This will help protect the environment.
6. Contents of the pack and other information
What Lanreotide Zentiva contains
The active substance is:
lanreotide 60 mg, 90 mg or 120 mg
The other ingredients are:
water for injections
acetic acid (to adjust pH)
What Lanreotide Zentiva looks like and contents of the pack
Lanreotide Zentiva is a thick solution for injection in a pre-filled syringe with an automatic safety system. The medicine is a semi-solid substance of white to light yellow color.
Each pre-filled syringe is packaged in a laminated pouch and a carton.
Pack sizes:
Carton with one 0.5 mL pre-filled syringe with automatic safety system and one needle (1.2 mm x 20 mm).
Carton with three pouches, each containing one 0.5 mL pre-filled syringe and one needle (1.2 mm x 20 mm).
1 pre-filled syringe of 0.5 mL (60 mg), 3 pre-filled syringes of 0.5 mL (60 mg)
1 pre-filled syringe of 0.5 mL (90 mg), 3 pre-filled syringes of 0.5 mL (90 mg)
1 pre-filled syringe of 0.5 mL (120 mg), 3 pre-filled syringes of 0.5 mL (120 mg)
Not all pack sizes may be marketed.
Marketing authorization holder:
Zentiva, k.s.
U kabelovny 130
Dolní Měcholupy
102 37 Prague 10
Czech Republic
Importer:
Terapia S.A.
124 Fabricii Street
400632 Cluj-Napoca
Romania
Sun Pharmaceutical Industries Europe B.V.
Polarisavenue 87
2132 JH Hoofddorp
Netherlands
This medicine is authorized in the Member States of the European Economic Area under the following names:
Czech Republic, Denmark, Finland, Norway, Sweden: Lanreotid Zentiva
Poland, Italy: Lanreotide Zentiva
Germany: Lanreotid Zentiva 60 mg, 90 mg, 120 mg Injektionslösung in einer Fertigspritze
Estonia: Lanreotida Zentiva 60 mg, 90 mg, 120 mg solución inyectable en jeringa precargada EFG
France: LANREOTIDE ZENTIVA LP 60 mg, 90 mg, 120 mg solution injectable à libération prolongée en seringue préremplie
Romania: Lanreotidă Zentiva 120 mg soluţie injectabilă în seringă preumplută
Slovakia: Lanreotid Zentiva 60 mg, 120 mg injekčný roztok v naplnenej injekčnej striekačke
For further information, contact the local representative of the marketing authorization holder:
Zentiva Polska Sp. z.o.o.
Bonifraterska 17 Street
00-203 Warsaw, Poland
phone: +48 22 375 92 00
Date of last revision of the leaflet:
- Country of registration
- Active substance
- Prescription requiredYes
- ImporterSun Pharmaceutical Industries Europe B.V. Terapia S.A.
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to Lanreotide ZentivaDosage form: Solution, 60 mgActive substance: lanreotidePrescription requiredDosage form: Solution, 120 mgActive substance: lanreotidePrescription requiredDosage form: Solution, 60 mgActive substance: lanreotideManufacturer: Pharmathen International S.A. Pharmathen S.A.Prescription required
Alternatives to Lanreotide Zentiva in other countries
The best alternatives with the same active ingredient and therapeutic effect.
Alternative to Lanreotide Zentiva in Spain
Online doctors for Lanreotide Zentiva
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for Lanreotide Zentiva – subject to medical assessment and local rules.






