
FYREMADEL 0.25 MG / 0.5 ML INJECTABLE SOLUTION IN PRE-FILLED SYRINGE EFG


How to use FYREMADEL 0.25 MG / 0.5 ML INJECTABLE SOLUTION IN PRE-FILLED SYRINGE EFG
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the User
Fyremadel0.25 mg/0.5 ml solution for injection in pre-filled syringe EFG
Ganirelix
Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor, pharmacist, or nurse.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor, pharmacist, or nurse. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the pack and other information
- What is Fyremadel and what is it used for
- What you need to know before you use Fyremadel
- How to use Fyremadel
- Possible side effects
- Storage of Fyremadel
Contents of the pack and further information
1. What is Fyremadel and what is it used for
Fyremadel contains the active substance ganirelix and belongs to a group of medicines called “gonadotropin-releasing hormone antagonists” that acts against the action of the endogenous gonadotropin-releasing hormone (GnRH). GnRH regulates the release of gonadotropins (luteinizing hormone (LH) and follicle-stimulating hormone (FSH)). The gonadotropins play an important role in human fertility and reproduction. FSH is necessary in women for the growth and development of follicles in the ovaries. The follicles are small, rounded vesicles that contain the eggs. LH is necessary for the release of mature eggs from the ovarian follicles (i.e., ovulation). Fyremadel inhibits the action of GnRH, which causes the suppression of the release of LH in particular.
What Fyremadel is used for
In women undergoing assisted reproduction techniques, such as in-vitro fertilization (IVF) and other methods, premature ovulation may occasionally occur, which significantly reduces the probability of becoming pregnant. Fyremadel is used to prevent the premature release of LH, which can cause premature ovulation.
In clinical studies, Fyremadel was used with recombinant follicle-stimulating hormone (FSH) or with corifollitropin alfa, a long-acting follicular stimulant.
2. What you need to know before you use Fyremadel
Do not useFyremadel
- if you are allergic to ganirelix or any of the other ingredients of this medicine (listed in section 6);
- if you are hypersensitive to gonadotropin-releasing hormone (GnRH) or its analogs;
- if you have moderate or severe kidney or liver disease;
- if you are pregnant or breastfeeding.
Warnings and precautions
Consult your doctor, pharmacist, or nurse before starting treatment with Fyremadel:
- If you have an active allergy, inform your doctor. Your doctor will decide, depending on the severity, if additional monitoring is needed during treatment. Allergic reactions have been observed, even after the first dose.
- Allergic reactions, both generalized and localized, including hives (urticaria), swelling of the face, lips, tongue, and/or throat that can cause difficulty breathing and/or swallowing (angioedema and/or anaphylaxis) (see also section 4). If you have an allergic reaction, stop using Fyremadel and seek medical attention immediately.
- Latex allergy: the needle shield contains dry natural rubber/latex, which may cause allergic reactions when in contact with the needle.
- During or after hormonal stimulation of the ovaries, ovarian hyperstimulation syndrome (OHSS) may develop. This syndrome is related to the gonadotropin stimulation procedure. We recommend that you read the package leaflet of the gonadotropin medicine that you have been prescribed.
- The incidence of congenital malformations after the use of assisted reproduction techniques may be slightly higher than after spontaneous conceptions. It is considered that this slightly higher incidence is related to the characteristics of patients undergoing fertility treatments (e.g., woman's age, semen characteristics) and the higher incidence of multiple pregnancies recorded after the use of assisted reproduction techniques. The incidence of congenital malformations after the use of assisted reproduction techniques with Fyremadel is not different from the incidence with the use of other GnRH analogs in assisted reproduction techniques.
- There is a slight increase in the risk of an ectopic pregnancy (a pregnancy that develops outside the uterus) in women with damaged Fallopian tubes.
- The efficacy and safety of Fyremadel have not been established in women who weigh less than 50 kg or more than 90 kg. Consult your doctor for more information.
Children and adolescents
Fyremadel is not intended for use in children or adolescents.
Using Fyremadel with other medicines
Tell your doctor or pharmacist if you are using, have recently used, or might use any other medicines.
Pregnancy, breastfeeding, and fertility
Fyremadel should be used during controlled ovarian stimulation for assisted reproduction techniques (ART). Do not use Fyremadel during pregnancy and breastfeeding.
If you are pregnant or breastfeeding, think you may be pregnant, or are planning to have a baby, ask your doctor or pharmacist for advice before using this medicine.
Driving and using machines
No studies on the effects on the ability to drive and use machines have been performed.
Fyremadel contains sodium
This medicine contains less than 1 mmol of sodium (23 mg) per injection, which is essentially "sodium-free".
3. How to use Fyremadel
Fyremadel is used as part of the treatment in assisted reproduction techniques (ART), including in-vitro fertilization (IVF).
You will administer the injections yourself, so your doctor should explain how to do it. Follow exactly the administration instructions of this medicine indicated by your doctor or pharmacist. If in doubt, consult your doctor or pharmacist again.
Phase 1
Ovarian stimulation with follicle-stimulating hormone (FSH) or corifollitropin alfa may start on the second or third day of your menstruation.
Phase 2
The contents of the Fyremadel syringe (0.25 mg) should be injected once a day just under the skin, starting on the fifth or sixth day of stimulation. Depending on your ovarian response, your doctor may decide to start on another day.
Fyremadel and FSH should be administered approximately at the same time. However, these medicines should not be mixed and should be injected in different locations.
Daily treatment with Fyremadel should continue until there are enough follicles of adequate size.
Phase 3
The final maturation of the eggs in the follicles can be induced by administering human chorionic gonadotropin (hCG). The time between two Fyremadel injections and between the last Fyremadel injection and the hCG injection should not exceed 30 hours; otherwise, premature ovulation (i.e., release of the eggs) may occur. Therefore, if the Fyremadel injection is given in the morning, Fyremadel treatment should be maintained throughout the entire gonadotropin treatment period, including the day of ovulation induction. If the Fyremadel injection is given in the afternoon, the last Fyremadel injection should be administered on the evening before the day of ovulation induction.
Instructions for use
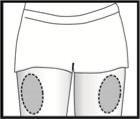
Injection site
Fyremadel is presented in pre-filled syringes that contain a dose. The contents of the syringe should be injected slowly just under the skin, preferably in the thigh. Check the solution before use. Do not use the solution if it contains particles or is not transparent. If you or your partner administer the injections, follow the instructions below carefully. Do not mix Fyremadel with other medicines.
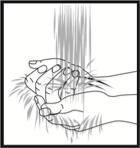
Preparing the injection site
Wash your hands thoroughly with water and soap. The injection site should be cleaned with a disinfectant (e.g., alcohol) to remove bacteria from the surface. Clean an area of about 5 cm around the injection point and let the disinfectant dry for at least one minute before injecting.
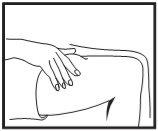
Inserting the needle
Remove the needle cap. Pinch a large area of skin between your index and thumb fingers. Insert the needle into the base of the pinched skin at an angle of 45° with respect to the skin surface. The injection site should be varied for each injection.
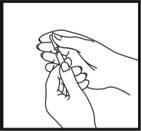
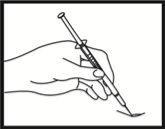
Checking the correct position of the needle
Gently pull back the plunger to check if the needle is correctly positioned. If blood enters the syringe, it means that the needle tip has penetrated a blood vessel. If this happens, do not inject Fyremadel; instead, remove the syringe, cover the injection site with a swab with disinfectant, and press; it should stop bleeding within one or two minutes. Do not use this syringe and dispose of it properly. Start again with a new syringe.
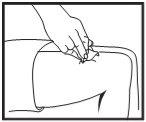
Injecting the solution
Once the needle is correctly positioned, press the plunger slowly and constantly to inject the solution correctly and avoid damaging the skin tissues.
Removing the syringe
Remove the syringe quickly and press on the injection site with a swab with disinfectant.
Use the pre-filled syringe only once.
If you use more Fyremadel than you should
Consult your doctor. In case of overdose or accidental ingestion, consult your doctor or pharmacist immediately or call the Toxicology Information Service, phone: 91 562 04 20, indicating the medicine and the amount taken.
If you forget to use Fyremadel
If you realize you have forgotten to inject a dose, administer it as soon as possible.
Do not take a double dose to make up for forgotten doses.
If you delay more than 6 hours (i.e., the interval between two injections is prolonged more than 30 hours), administer the dose as soon as possible andconsult your doctor for advice.
If you stop using Fyremadel
Do not stop using Fyremadel unless your doctor tells you to, as this may affect the outcome of your treatment.
If you have any further questions on the use of this medicine, ask your doctor, pharmacist, or nurse.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Very common (may affect up to 1 in 10 women)
- local reactions at the injection site (mainly redness, with or without swelling). The local reaction usually disappears within 4 hours after administration.
Uncommon (may affect up to 1 in 100 women)
- headache
- nausea
- malaise (general feeling of being unwell, feeling sick).
Very rare (may affect up to 1 in 10,000 women)
- allergic reactions have been observed, even after the first dose, including rash
- facial swelling
- difficulty breathing (dyspnea)
- swelling of the face, lips, tongue, and/or throat, which can cause difficulty breathing and/or swallowing (angioedema and/or anaphylaxis)
- hives (urticaria).
In addition, side effects related to controlled ovarian hyperstimulation treatment have been observed, such as:
- abdominal pain,
- ovarian hyperstimulation syndrome (OHSS), (OHSS occurs when the ovaries react excessively to the fertility medicines you are taking)
- ectopic pregnancy (when the embryo develops outside the uterus)
- miscarriage (see the package leaflet of the medicine with FSH that you are using).
After the first dose of Fyremadel, worsening of a pre-existing eczema has been reported in one patient.
Reporting of side effects
If you experience any side effects, talk to your doctor, pharmacist, or nurse. This includes any possible side effects not listed in this leaflet. You can also report side effects directly to the Spanish Medicines Agency (AEMPS) at https://www.notificaram.es.
By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of Fyremadel
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date which is stated on the carton after ‘EXP’. The expiry date refers to the last day of that month.
This medicine does not require any special storage conditions.
Inspect the syringe before use. The syringe should only be used if the solution is transparent and particle-free and the container is not damaged.
Medicines should not be disposed of via wastewater or household waste. Return any unused medicine to a pharmacy for proper disposal. If you are unsure, ask your pharmacist how to dispose of unused medicines. This will help protect the environment.
6. Contents of the pack and further information
Composition of Fyremadel
- The active substance is ganirelix. Each pre-filled syringe contains 0.25 mg of ganirelix (as acetate) in 0.5 ml of aqueous solution.
- The other ingredients are glacial acetic acid (E260), mannitol (E421), and water for injections. The pH may have been adjusted with sodium hydroxide and glacial acetic acid.
Appearance and pack contents
Fyremadel is a clear and colorless aqueous solution for injection. The solution is ready to use and is administered subcutaneously. The needle shield contains dry natural rubber/latex, which is in contact with the needle.
Fyremadel is available in packs of 1 or 5 pre-filled syringes with injection needles (27 G).
Not all pack sizes may be marketed.
Marketing authorization holder and manufacturer
Sun Pharmaceutical Industries Europe B.V.
Polarisavenue 87
2132 JH Hoofddorp
Netherlands
You can obtain further information on this medicine from the representative of the marketing authorization holder:
FERRING S.A.U.
C/Arquitecto Sánchez Arcas nº 3 1º28040, Madrid, Spain
This medicine is authorized in the Member States of the European Economic Area under the following names:
Austria: Fyremadel 0,25 mg/0,5 ml Injektionslösung in einer Fertigspritze
Denmark: Fyremadel 0,25 mg/0,5 ml injektionsvæske, opløsning i fyldt injektionssprøjte
Spain: Fyremadel 0,25 mg/0,5 ml solución inyectable en jeringa precargada EFG
Finland: Fyremadel 0,25 mg/0,5 ml injektioneste, liuos esitäytetty ruisku
France: Fyremadel 0,25 mg/0,5 ml solution injectable en seringue pré-remplie
Germany: Fyremadel 0,25 mg/0,5 ml Injektionslösung in einer Fertigspritze
Italy: Fyremadel 0,25 mg/0,5 ml soluzione iniettabile in siringa preriempita
Netherlands: Fyremadel 0,25 mg/0,5 ml oplossing voor injectie in voorgevulde spuit
Norway: Fyremadel 0,25 mg/0,5 ml injeksjonsvæske, oppløsning, i ferdigfylt sprøyte
Sweden: Fyremadel 0,25 mg/0,5 ml injektionsvätska, lösning, förfylld spruta
United Kingdom: Fyremadel 0.25 mg/0.5 ml solution for injection in pre-filled syringe
Date of last revision of this leaflet:April 2020
Detailed and up-to-date information on this medicine is available on the website of the Spanish Agency for Medicines and Health Products (AEMPS) http://www.aemps.gob.es/.
- Country of registration
- Availability in pharmacies
Supply issue reported
Data from the Spanish Agency of Medicines (AEMPS) indicates a supply issue affecting this medicine.<br><br>Availability may be limited in some pharmacies.<br><br>For updates or alternatives, consult your pharmacist. - Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to FYREMADEL 0.25 MG / 0.5 ML INJECTABLE SOLUTION IN PRE-FILLED SYRINGE EFGDosage form: INJECTABLE, 0.25 mg/0.5 mlActive substance: ganirelixManufacturer: Gp Pharm S.A.Prescription requiredDosage form: INJECTABLE, 0.25 mg/0.5 mLActive substance: ganirelixManufacturer: Gedeon Richter Plc.Prescription requiredDosage form: INJECTABLE, UnknownActive substance: ganirelixManufacturer: Organon N.V.Prescription required
Online doctors for FYREMADEL 0.25 MG / 0.5 ML INJECTABLE SOLUTION IN PRE-FILLED SYRINGE EFG
Discuss questions about FYREMADEL 0.25 MG / 0.5 ML INJECTABLE SOLUTION IN PRE-FILLED SYRINGE EFG, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions






