
GANIRELIX GEDEON RICHTER 0.25 mg/0.5 ml Injectable Solution in Pre-filled Syringe EFG

How to use GANIRELIX GEDEON RICHTER 0.25 mg/0.5 ml Injectable Solution in Pre-filled Syringe EFG
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the User
Ganirelix Gedeon Richter 0.25 mg/0.5 ml solution for injection in pre-filled syringe
ganirelix
Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor, pharmacist or nurse.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor, pharmacist or nurse. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the pack
- What is Ganirelix Gedeon Richter and what is it used for
- What you need to know before you use Ganirelix Gedeon Richter
- How to use Ganirelix Gedeon Richter
- Possible side effects
- Storage of Ganirelix Gedeon Richter
- Contents of the pack and other information
1. What is Ganirelix Gedeon Richter and what is it used for
Ganirelix Gedeon Richter contains the active substance ganirelix and belongs to a group of medicines called “gonadotropin-releasing hormone antagonists” that act against the action of endogenous gonadotropin-releasing hormone (GnRH). GnRH regulates the release of gonadotropins (luteinizing hormone (LH) and follicle-stimulating hormone (FSH)). Gonadotropins play an important role in human fertility and reproduction. FSH is necessary in women for the growth and development of follicles in the ovaries. The follicles are small, rounded vesicles that contain the eggs. LH is necessary for the release of mature eggs from the ovarian follicles (i.e., ovulation). This medicine inhibits the action of GnRH, which causes the suppression of the release of LH in particular.
What Ganirelix Gedeon Richter is used for
In women undergoing assisted reproduction techniques, such as in vitro fertilization (IVF) and other methods, premature ovulation may occasionally occur, which significantly reduces the likelihood of becoming pregnant. This medicine is used to prevent the premature release of LH, which can cause premature ovulation.
In clinical studies, ganirelix was used with recombinant follicle-stimulating hormone (FSH) or with corifollitropin alfa, a long-acting follicular stimulant.
2. What you need to know before you use Ganirelix Gedeon Richter
Do not use Ganirelix Gedeon Richter
- if you are allergic to ganirelix or any of the other ingredients of this medicine (listed in section 6);
- if you are hypersensitive to gonadotropin-releasing hormone (GnRH) or its analogues;
- if you have moderate or severe kidney or liver disease;
- if you are pregnant or breastfeeding.
Warnings and precautions
Consult your doctor, pharmacist or nurse before starting treatment with Ganirelix Gedeon Richter.
Allergic reactions
If you have an active allergy, inform your doctor. Your doctor will decide, depending on the severity, if additional controls are needed during treatment. Allergic reactions have been observed, even after the first dose.
Allergic reactions, both generalized and localized, have been reported, including hives (urticaria), swelling of the face, lips, tongue and/or throat that can cause difficulty breathing and/or swallowing (angioedema and/or anaphylaxis) (see also section 4). If you have an allergic reaction, stop using Ganirelix Gedeon Richter and seek medical attention immediately.
Ovarian Hyperstimulation Syndrome (OHSS)
During or after hormonal stimulation of the ovaries, Ovarian Hyperstimulation Syndrome may develop. This syndrome is related to the gonadotropin stimulation procedure. We recommend that you read the package leaflet of the gonadotropin medicine that you have been prescribed.
Multiple births or birth defects
The incidence of congenital malformations after the use of assisted reproduction techniques may be slightly higher than after spontaneous conceptions. This slightly higher incidence is considered to be related to the characteristics of patients undergoing fertility treatments (e.g., woman's age, semen characteristics) and to the higher incidence of multiple pregnancies recorded after the use of assisted reproduction techniques. The incidence of congenital malformations after the use of assisted reproduction techniques with ganirelix is not different from the incidence with the use of other GnRH analogues in assisted reproduction techniques.
Pregnancy complications
There is a slight increase in the risk of an ectopic pregnancy (a pregnancy outside the uterus) in women with damaged Fallopian tubes.
Women weighing less than 50 kg or more than 90 kg
The efficacy and safety of ganirelix have not been established in women weighing less than 50 kg or more than 90 kg. Consult your doctor for more information.
Children and adolescents
Ganirelix Gedeon Richter is not intended for use in children or adolescents.
Using Ganirelix Gedeon Richter with other medicines
Tell your doctor or pharmacist if you are using, have recently used or might use any other medicines.
Pregnancy, breastfeeding and fertility
Ganirelix Gedeon Richter should be used during controlled ovarian stimulation for assisted reproduction techniques (ART).
Do not use Ganirelix Gedeon Richter during pregnancy and breastfeeding.
Consult your doctor or pharmacist before using this medicine.
Driving and using machines
The effects of Ganirelix Gedeon Richter on the ability to drive and use machines have not been studied.
Ganirelix Gedeon Richter contains sodium
This medicine contains less than 1 mmol of sodium (23 mg) per injection, i.e., it is essentially “sodium-free”.
3. How to use Ganirelix Gedeon Richter
Follow exactly the administration instructions of this medicine given by your doctor or pharmacist. If you are in doubt, consult your doctor or pharmacist again.
Ganirelix Gedeon Richter is used as part of the treatment in assisted reproduction techniques (ART), including in vitro fertilization (IVF).
Ovarian stimulation with follicle-stimulating hormone (FSH) or with corifollitropin alfa may start on the second or third day of your menstrual period. Ganirelix Gedeon Richter (0.25 mg) should be injected into the fatty layer just under the skin once a day, starting on the fifth or sixth day of stimulation. Depending on your ovarian response, your doctor may decide to start on another day.
Ganirelix Gedeon Richter and FSH should be administered approximately at the same time. However, these medicines should not be mixed and should be injected into different areas.
Daily treatment with Ganirelix Gedeon Richter should continue until there are enough follicles of adequate size. Final maturation of the eggs in the follicles can be induced by administering human chorionic gonadotropin (hCG). The time between two injections of Ganirelix Gedeon Richter and the injection of hCG should not exceed 30 hours, otherwise premature ovulation (i.e., release of eggs) may occur.
Therefore, if the injection of Ganirelix Gedeon Richter is in the morning, treatment with Ganirelix Gedeon Richter should be maintained throughout the entire treatment period with gonadotropin, including the day of ovulation induction.
If the injection of Ganirelix Gedeon Richter is in the afternoon, the last injection of Ganirelix Gedeon Richter should be administered on the evening before the day of ovulation induction.
Instructions for use
Before administering this medicine, it is very important that you also read carefully and follow exactly the detailed instructions for use provided at the end of this leaflet.
Injection site
Ganirelix Gedeon Richter is presented in pre-filled syringes and should be injected slowly just under the skin, preferably in the thigh. Check the solution before use. Do not use the solution if it contains particles or is not transparent. You may notice air bubbles in the pre-filled syringe. This is to be expected and it is not necessary to remove the air bubbles. If you or your partner administer the injections, follow exactly the instructions that appear below and at the end of the leaflet. Do not mix Ganirelix Gedeon Richter with other medicines.
Preparing the injection site
Wash your hands thoroughly with water and soap. The injection site should be cleaned with a disinfectant (e.g., alcohol) to remove bacteria from the surface. Clean an area of about 5 cm around the injection point and let the disinfectant dry for at least one minute before injecting.
Inserting the needle
Remove the needle protector. Pinch a large area of skin between your index and thumb fingers. Insert the needle into the base of the pinched skin at an angle of 45° with respect to the skin surface. The injection site should be varied for each injection.
Checking the correct position of the needle
Gently pull back the plunger to check if the needle is correctly positioned. If blood enters the syringe, it means that the needle tip has penetrated a blood vessel. If this happens, do not inject Ganirelix Gedeon Richter, but remove the syringe, cover the injection site with a swab with disinfectant and press; it should stop bleeding within one or two minutes. Do not use this syringe and dispose of it properly. Start again with a new syringe.
Injecting the solution
Once the needle is correctly positioned, press the plunger slowly and constantly to inject the solution correctly and avoid damaging the skin tissues. Press the plunger until the syringe is empty and wait 5 seconds.
Removing the syringe
Remove the syringe quickly and press on the injection site with a swab with disinfectant. Use the pre-filled syringe only once.
If you use more Ganirelix Gedeon Richter than you should
Consult your doctor.
If you forget to use Ganirelix Gedeon Richter
If you realize that you have forgotten to inject a dose, administer it as soon as possible. Do not administer a double dose to make up for forgotten doses.
If you delay more than 6 hours (i.e., the interval between two injections is prolonged more than 30 hours), administer the dose as soon as possible and consult your doctor for advice.
If you stop treatment with Ganirelix Gedeon Richter
Do not stop using Ganirelix Gedeon Richter unless your doctor tells you to, as this may affect the outcome of your treatment.
If you have any further questions on the use of this medicine, ask your doctor, pharmacist or nurse.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
The likelihood of experiencing a side effect is classified into the following categories:
Very common (may affect more than 1 in 10 women)
- Local reactions at the injection site (mainly redness, with or without swelling). The local reaction usually disappears within 4 hours after administration.
Uncommon (may affect up to 1 in 100 women)
- Headache
- Nausea
- Feeling unwell (malaise)
Very rare (may affect up to 1 in 10,000 women)
- Allergic reactions have been observed, even after the first dose.
- Rash
- Facial swelling
- Difficulty breathing (dyspnea)
- Swelling of the face, lips, tongue and/or throat, which can cause difficulty breathing and/or swallowing (angioedema and/or anaphylaxis)
- Hives (urticaria)
- Worsening of a pre-existing eczema has been reported after the first dose of ganirelix.
Additionally, side effects related to controlled ovarian hyperstimulation treatment have been observed (such as abdominal pain, Ovarian Hyperstimulation Syndrome (OHSS), ectopic pregnancy (when the embryo develops outside the uterus) and abortion (see the package leaflet of the gonadotropin medicine you are using).
Reporting of side effects
If you experience any side effects, talk to your doctor, pharmacist or nurse. This includes any possible side effects not listed in this leaflet. You can also report side effects directly through the national reporting system listed in Appendix V. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of Ganirelix Gedeon Richter
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date which is stated on the label and carton after EXP. The expiry date is the last day of the month stated.
Do not freeze.
Store in the original package to protect from light.
Inspect the syringe before use. The syringe should only be used if the solution is transparent and particle-free and the container is not damaged.
Each pre-filled syringe is for single use only.
For the administration of this medicine, alcohol swabs, gauzes, and a container for sharp objects are needed, but they are not included in the packaging.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. This will help protect the environment.
6. Container Contents and Additional Information
Composition of Ganirelix Gedeon Richter
- The active ingredient is ganirelix (0.25 mg in 0.5 ml of solution).
- The other components are: glacial acetic acid, mannitol (E 421), water for injectable preparations. The pH (measure of acidity) may have been adjusted with sodium hydroxide (see section 2 "Ganirelix Gedeon Richter contains sodium").
Appearance of Ganirelix Gedeon Richter and Container Contents
Ganirelix Gedeon Richter is a clear and colorless injectable solution. The medication is packaged in a glass syringe with a stainless steel needle, closed with a plunger stopper and a plunger rod. The injection needle is provided with a rigid protector.
Ganirelix Gedeon Richter is available in packs of 1 or 6 pre-filled syringes.
Only some pack sizes may be marketed.
Marketing Authorization Holder and Manufacturer
Gedeon Richter Plc.
Gyömroi út 19-21.
1103 Budapest
Hungary
Date of Last Revision of this Leaflet:
Other Sources of Information
Detailed information on this medication is available on the European Medicines Agency website: http://www.ema.europa.eu
Instructions for Use
HOW TO PREPARE AND ADMINISTER GANIRELIX GEDEON RICHTER IN A PRE-FILLED SYRINGE
Before administering this medication, read these instructions and the entire leaflet carefully.
These instructions will help you learn how to administer the Ganirelix Gedeon Richter injection to yourself or your partner. If you are unsure about administering the injection or have any doubts, consult your doctor or pharmacist.
Do not mix Ganirelix Gedeon Richter with any other medication.
Administer the injection at the same time every day.
Each pre-filled syringe contains a daily dose of ganirelix.
Each pre-filled syringe contains a daily dose of ganirelix.
CONTENTS OF THE INSTRUCTIONS FOR USE
- Preparation of the injection
- Preparation of the injection site
- Administration of the medication
- After the injection
- Preparation of the injection
- Wash your hands thoroughly with water and soap. It is essential that your hands and the utensils you use are as clean as possible to avoid infections.
- Find a clean area to place the utensils you need for the injection, e.g., a clean table or a similar horizontal surface.
- Gather everything you need and place it in the clean area:
- Swabs with disinfectant (e.g., alcohol)
- 1 pre-filled syringe with the medication
- Do not hold the syringe by the plunger to avoid disassembling it.
- Do not use this medication after the expiration date shown on the label and carton after EXP. The expiration date is the last day of the month indicated.
- A container for sharp objects (e.g., a plastic bottle with a sufficiently large opening) to safely dispose of the used syringe.
- Inspect the solution before using it.
- You may see air bubbles in the pre-filled syringe. This is expected, and it is not necessary to remove the air bubble(s).
- Do not use the syringe if:
- it is cracked or damaged, or
- the needle protector has been removed or is not properly placed, or
- a liquid leak is observed, or
- the solution appears abnormal (contains particles or is not colorless).
If any of these situations occur, you must safely discard the syringe in the sharp objects container and use another one.
- Preparation of the injection site
- Choose the injection site, preferably on the thigh. You should vary the injection site to prevent damaging the tissue under the skin.
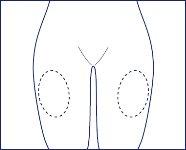
- Do not inject into any sensitive, damaged, or bruised area. Always choose an area of intact skin to inject the medication.
- Do not inject through clothing.
- Administer Ganirelix Gedeon Richter and the follicle-stimulating hormone (FSH) approximately at the same time. However, these medications should not be mixed and should be administered at different sites.
- Clean the injection site with a disinfectant (e.g., alcohol) at the chosen injection point to eliminate any superficial bacteria. Clean an area of about 5 cm (2 inches) in diameter around the point where the needle will enter and let the disinfectant dry for approximately 1 minute before proceeding.
- Do not touch the injection site or blow on it before the injection.
- Administration of the medication
- You will receive Ganirelix Gedeon Richter in pre-filled syringes with an integrated needle, ready for administration without the need for further adjustments to the syringe.
- Remove 1 syringe from the packaging by holding it by the middle of the syringe body.
- Remove the needle protector from the syringe while keeping it in a horizontal position and pointing away from you. Pull the needle protector to remove it, do not twist it.
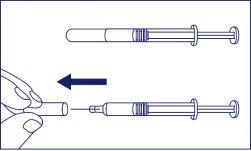
- When handling the syringe, do not touch the tip of the syringe or the needle with your fingers to avoid contamination.
- In the chosen and disinfected injection site, pinch a skin fold between your index and thumb fingers.
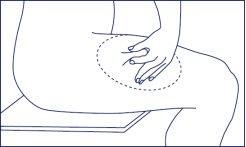
- Administer Ganirelix Gedeon Richter subcutaneously, i.e., in the fatty layer just under the skin.
- Hold the syringe with your other hand to be able to place your thumb on the plunger when necessary. Insert the needle completely with a quick and firm movement in the center of the skin fold at an angle of 45 degrees relative to the skin surface.
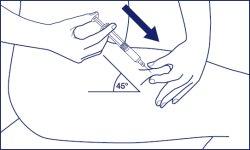
- Gently pull back the plunger to check if the needle is correctly placed.
- If blood enters the syringe, it means that the needle has penetrated a blood vessel. If this occurs, do not inject Ganirelix Gedeon Richter, remove the syringe, cover the injection site with a swab with disinfectant, and press; the bleeding should stop in one or two minutes.
- Do not use this syringe; dispose of it properly and use a new one.
- Once the needle is correctly placed, push the plunger down with your thumb slowly and steadily, so that the solution is injected correctly and the skin tissues are not damaged.
- Push the plunger down until the syringe is empty.
- After the injection
- Wait 5 seconds (count slowly to 5), then release the pinched skin.
- Quickly remove the syringe from your skin and apply pressure to the injection site with a swab impregnated with disinfectant.
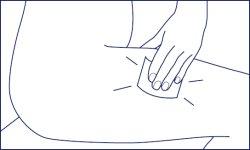
- Do not rub the skin after the injection.
- Use each pre-filled syringe only once.
- Do not recap the needle to avoid a possible puncture.
- Immediately dispose of the used syringe safely in the sharp objects container and return it to the pharmacy for proper disposal. Ask your pharmacist how to dispose of unused medications.
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to GANIRELIX GEDEON RICHTER 0.25 mg/0.5 ml Injectable Solution in Pre-filled Syringe EFGDosage form: INJECTABLE, 0.25 mg/0.5 mlActive substance: ganirelixManufacturer: Gp Pharm S.A.Prescription requiredDosage form: INJECTABLE, 0.25 mg/0.5 mgActive substance: ganirelixManufacturer: Sun Pharmaceutical Industries (Europe) B.V.Prescription requiredDosage form: INJECTABLE, UnknownActive substance: ganirelixManufacturer: Organon N.V.Prescription required
Online doctors for GANIRELIX GEDEON RICHTER 0.25 mg/0.5 ml Injectable Solution in Pre-filled Syringe EFG
Discuss questions about GANIRELIX GEDEON RICHTER 0.25 mg/0.5 ml Injectable Solution in Pre-filled Syringe EFG, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions






