

Oftahist

Ask a doctor about a prescription for Oftahist

How to use Oftahist
Package Leaflet: Information for the User
Oftahist, 1 mg/ml, eye drops, solution
Olopatadine
Read the package leaflet carefully before using the medicine, as it contains important information for the patient.
This medicine should always be used exactly as described in the package leaflet or as directed by a doctor or pharmacist.
- Keep this package leaflet, you may need to read it again.
- If you need advice or further information, consult a pharmacist.
- If you experience any side effects, including any not listed in this leaflet, tell your doctor or pharmacist. See section 4.
- If after 3 days there is no improvement or you feel worse, consult a doctor.
Table of Contents of the Package Leaflet
- 1. What is Oftahist and what is it used for
- 2. Important information before using Oftahist
- 3. How to use Oftahist
- 4. Possible side effects
- 5. How to store Oftahist
- 6. Contents of the pack and other information
1. What is Oftahist and what is it used for
Oftahist is intended for the treatment of eye symptoms (itching, tearing, redness, swelling of the eyelids and conjunctiva) in patients with diagnosed seasonal allergic conjunctivitis.
Oftahist may only be used in adults.
Allergic conjunctivitis.Certain substances (allergens), such as plant pollen, house dust or animal dander, can cause allergic reactions, manifested by itching, redness, tearing, and swelling of the eye surface. These symptoms occur suddenly, are acute and transient. They are often accompanied by watery rhinitis and sneezing or itching in the nose and ears.
Oftahist is a medicineused to treat allergic eye diseases. Its action is based on reducing the severity of allergic reactions.
2. Important information before using Oftahist
When not to use Oftahist:
- if the patient is allergic to olopatadine or any of the other ingredients of this medicine (listed in section 6).
Warnings and precautions
Consult a doctor if:
- there is uncertainty whether the symptoms are caused by an allergy. This applies in particular to situations where the symptoms affect only one eye, there are vision disturbances, or eye pain without nasal symptoms.
- the symptoms worsen or do not improve after 72 hours, despite using Oftahist eye drops.
Remove contact lenses before using Oftahist.
Children and adolescents
Oftahist should not be used in children and adolescents under 18 years of age.
Oftahist and other medicines
Tell your doctor or pharmacist about all medicines you are taking, have recently taken, or plan to take.
If you are using other eye drops or ointments, keep a minimum of 5-10 minutes between administrations of consecutive medicines. Eye ointments should be used last.
Pregnancy, breastfeeding, and fertility
If you are pregnant or breastfeeding, think you may be pregnant, or plan to
have a child, consult a doctor before using this medicine.
Do not use Oftahist during pregnancy and breastfeeding.
Driving and using machines
For some time after administering Oftahist, vision may be blurred.
Do not drive vehicles or operate machines until this symptom subsides.
Oftahist contains benzalkonium chloride
Benzalkonium chloride may cause eye irritation and is known to cause discoloration of soft contact lenses, so avoid contact with soft contact lenses. If you use contact lenses, remove them before using this medicine and wait 15 minutes before reinserting the lenses.
3. How to use Oftahist
This medicine should always be used exactly as described in the package leaflet or as directed by a doctor or pharmacist. If you are unsure, consult a doctor or pharmacist.
The medicine is applied locally to the conjunctival sac.
Before using the medicine, remove contact lenses and wait at least 15 minutes before reinserting them.
Adults
The recommended dose is one drop into the eye(s) twice a day - in the morning and evening. Keep a minimum of 8 hours between administrations.
If there is no improvement or symptoms worsen within three days of starting treatment, consult a doctor. The treatment duration is up to two weeks. If necessary, treatment can be continued for up to four months after consulting a doctor.
Do not use the medicine for more than 4 months.
Use in children and adolescents
Oftahist should not be used in children and adolescents under 18 years of age.
Instructions for use:
Oftahist should only be used as eye drops.
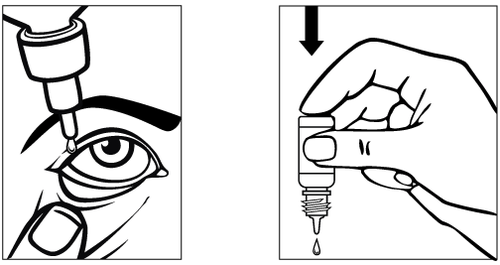
1. 2.
- Prepare the Oftahist bottle and a mirror.
- Wash your hands.
- Take the bottle in your hand and unscrew the cap.
- If the protective collar is loose after removing the cap, remove it before using the medicine.
- Hold the bottle with the tip down, using your thumb and middle finger.
- Tilt your head back. With a clean finger, pull the lower eyelid down to create a "pocket"; the drop should fall into it (diagram 1).
- Bring the bottle tip close to the eye. You can use a mirror to help.
- Do not touch the dropper to the eye or eyelid, or surrounding areas, as this may lead to infection of the remaining drops in the bottle.
- Gently squeeze the bottom of the bottle to release a single drop of Oftahist.
- Do not squeeze the bottle; it is designed so that gentle pressure on the bottom is enough to release a drop (diagram 2).
- If using drops in both eyes, repeat the steps for the second eye.
- Immediately after use, tighten the bottle cap.
If the drop does not fall into the eye, repeat the attempt.
Using more Oftahist than recommended
Rinse your eyes with lukewarm water. Do not administer the next drop until the scheduled time for the next dose.
Missing a dose of Oftahist
Administer one drop into the eye as soon as possible, and then return to the normal dosing schedule. If it is almost time for the next dose, skip the missed dose and continue with the scheduled dosing regimen.
If you are using other eye medicines, keep a minimum of five to ten minutes between administering Oftahist and other medicines.
Do not use a double dose to make up for a missed dose.
Stopping use of Oftahist
If you have any further questions about using the medicine, consult a doctor or pharmacist.
4. Possible side effects
Like all medicines, Oftahist can cause side effects, although not everybody gets them.
Stop treatment and contact a doctoror the Emergency Department of the nearest hospital if you experience any symptoms of hypersensitivity, including breathing difficulties, throat or face swelling.
In case of a severe allergic reaction, immediate emergency treatment may be necessary.
The following side effects have been observed during treatment with Oftahist:
Frequent(occurring in less than 1 in 10 patients)
Eye-related:eye pain, eye irritation, dry eye, unusual sensations in the eye.
General:headaches, unpleasant taste, dryness of the nasal mucosa, fatigue.
Uncommon(occurring in less than 1 in 100 patients)
Eye-related:corneal disorders, eye surface inflammation with or without damage, eye discharge, light sensitivity, blurred or impaired vision, eyelid spasms, eye discomfort, eye itching, conjunctival disorders, feeling of a foreign body in the eye, increased tear production, eyelid redness or swelling, eyelid abnormalities, eye redness.
General:nasal mucosa inflammation, dizziness, abnormal or impaired sensation, skin inflammation (contact), skin burning sensation, skin dryness.
Frequency not known(cannot be estimated from available data)
Eye-related:corneal edema, eye swelling, conjunctivitis, changes in pupil size, vision disturbances, eyelid margin crusts.
General:hypersensitivity, face swelling, drowsiness, shortness of breath, sinusitis, nausea, vomiting, skin inflammation and redness, general weakness, malaise.
In patients with significantly damaged anterior, transparent layer of the eye (cornea), very rare cases of cloudy spots on the cornea caused by calcium deposits during treatment have been observed.
Reporting side effects
If you experience any side effects, including those not listed in this leaflet, tell your doctor or pharmacist or nurse.
Side effects can be reported directly to the Department of Post-Marketing Surveillance of the Office for Registration of Medicinal Products, Medical Devices, and Biocidal Products, Al. Jerozolimskie 181C, 02-222 Warsaw, tel.: +48 22 49 21 301, fax +48 22 49 21 309, e-mail: [email protected]
Side effects can also be reported to the marketing authorization holder.
Reporting side effects will help gather more information on the safety of this medicine.
5. How to store Oftahist
Keep the medicine out of the sight and reach of children.
Do not use this medicine after the expiry date stated on the bottle and carton after "Exp". The expiry date refers to the last day of the month.
This medicine does not require any special storage conditions.
To prevent infections, the bottle should be discarded 4 weeks after first opening, and then use the next bottle of medicine. Record the opening date of the bottle on the label and carton.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. This will help protect the environment.
6. Contents of the pack and other information
What Oftahist contains
- The active substance is olopatadine. Each ml of solution contains 1 mg of olopatadine (as hydrochloride). The other ingredients are benzalkonium chloride, sodium chloride, anhydrous disodium phosphate, sodium hydroxide (to adjust pH), hydrochloric acid (to adjust pH), water for injections.
What Oftahist looks like and contents of the pack
Oftahist is a clear solution (solution) supplied in a pack containing a 5 ml plastic bottle with a screw cap.
Marketing authorization holder and manufacturer
Marketing authorization holder
Adamed Pharma S.A.
Pieńków, ul. M. Adamkiewicza 6A
05-152 Czosnów
Manufacturer/Importer
Mako Pharma Sp. z o.o.
ul. Wiśniowa 9
05-092 Kiełpin
Adamed Pharma S.A.
Pieńków, ul. M. Adamkiewicza 6A
05-152 Czosnów
This medicinal product is authorized in the Member States of the European Economic Area under the following names:
Poland
Oftahist
Date of last revision of the package leaflet:
- Country of registration
- Active substance
- Prescription requiredNo
- Manufacturer
- ImporterAdamed Pharma S.A. Mako Pharma Sp. z o.o. Pabianickie Zakłady Farmaceutyczne Polfa S.A.
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to OftahistDosage form: Drops, 1 mg/mlActive substance: olopatadineManufacturer: Rafarm S.A.Prescription requiredDosage form: Drops, 1 mg/mlActive substance: olopatadinePrescription requiredDosage form: Drops, 1 mg/mlActive substance: olopatadineManufacturer: Rafarm S.A.Prescription not required
Alternatives to Oftahist in other countries
The best alternatives with the same active ingredient and therapeutic effect.
Alternative to Oftahist in Spain
Alternative to Oftahist in Ukraine
Online doctors for Oftahist
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for Oftahist – subject to medical assessment and local rules.














