
OPATANOL 1 mg/ml EYE DROPS SOLUTION

How to use OPATANOL 1 mg/ml EYE DROPS SOLUTION
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the User
Opatanol 1mg/ml eye drops solution
olopatadine
Read the entire package leaflet carefully before startingto use thismedicine, as it contains important information for you.
- Keep this package leaflet, as you may need to read it again.
- If you have any questions, ask your doctor or pharmacist.
- This medicine has been prescribed to you only, and you should not give it to others, even if they have the same symptoms as you, as it may harm them.
- If you experience side effects, ask your doctor or pharmacist, even if they are side effects not listed in this package leaflet. See section 4.
Contents of the package leaflet
- What is Opatanol and what is it used for
- What you need to know before starting to use Opatanol
- How to use Opatanol
- Possible side effects
- Storage of Opatanol
- Package contents and additional information
1. What is Opatanol and what is it used for
Opatanolis indicated for the treatment of signs and symptoms of seasonal allergic conjunctivitis.
Allergic conjunctivitis.Some elements (called allergens) such as pollen, house dust, or animal hair can cause allergic reactions that lead to itching and redness as well as inflammation of the surface of your eyes.
Opatanol belongs to the group of medicines usedfor the treatment of allergic eye conditions. It works by reducing the intensity of the allergic reaction.
2. What you need to know before starting to use Opatanol
Do not useOpatanol
- If you are allergic(hypersensitive) to olopatadine or any of the other ingredients of this medicine (listed in section 6).
- Do not use Opatanol if you are breastfeeding.
Warnings and precautions
Consult your doctor or pharmacist before starting to use Opatanol.
You should remove your contact lenses before using Opatanol.
Children
Do not use Opatanol in children under 3 years of age. Do not administer this medicine to children under 3 years of age, as there is no data indicating that it is safe and effective in children under 3 years of age.
Other medicines and Opatanol
Tell your doctor or pharmacist if you are using, have recently used, or may need to use any other medicine.
If you are using another eye drop or ointment, wait at least 5 minutes between applying each medicine. Eye ointments should be applied last.
Pregnancy, breastfeeding, and fertility
If you are pregnant or breastfeeding, think you may be pregnant, or plan to become pregnant, consult your doctor or pharmacist before using this medicine.
Do not use Opatanol if you are breastfeeding. Consult your doctor before using this medicine.
Driving and using machines
Immediately after applying Opatanol, you may notice that your vision is blurred. Do not drive or use machines until this effect has disappeared.
Opatanolcontains benzalkonium chloride
This medicine contains 0.5 mg of benzalkonium chloride in every 5 ml, which is equivalent to 0.1 mg/ml.
The preservative of Opatanol, benzalkonium chloride, can be absorbed by soft contact lenses and may alter the color of the contact lenses. Remove your contact lenses before using this medicine and wait 15 minutes before putting them back in.
Benzalkonium chloride can cause eye irritation, especially if you have dry eyes or other corneal diseases (the transparent layer on the front of the eye). Consult your doctor if you feel any unusual sensation, itching, or pain in the eye after using this medicine.
Opatanol contains disodium phosphate dodecahydrate
This medicine contains 16.72 mg of phosphates (in 63.05 mg of disodium phosphate dodecahydrate) in every 5 ml, which is equivalent to 3.34 mg/ml.
If you have severe corneal damage (the transparent layer on the front of the eye), treatment with phosphates, in very rare cases, can cause blurred vision due to calcium accumulation.
3. How to use Opatanol
Follow the instructions for administration of this medicine indicated by your doctor or pharmacist. If you are unsure, consult your doctor or pharmacist again.
The recommended dose is one drop in one eye or both eyes, twice a day - in the morning and in the evening.
Use this amount unless your doctor gives you other instructions. You should only apply Opatanol to both eyes if your doctor has told you to do so. Follow the treatment for the period of time indicated by your doctor.
Opatanol should only be used as eye drops.
FOR MORE INFORMATION SEE BACK COVER
Turn the package leaflet over.
How to use Opatanol(continued)
1 2
Dose to use
See front of the package leaflet
- Take the Opatanol bottle and stand in front of a mirror.
- Wash your hands.
- Take the bottle and unscrew the cap.
- After removing the cap, you must remove the plastic ring from the seal before using.
- Hold the bottle, upside down, between your thumb and index finger.
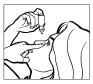
- Tilt your head back. Gently pull your lower eyelid down to form a pocket, where the drop should fall (Figure 1).
- Bring the tip of the bottle close to your eye. You may find it helpful to look in a mirror.
- Do not touch your eye, eyelid, or surrounding areas with the dropper, as the drops that remain in the bottle may become contaminated.
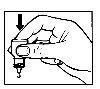
- Gently press the base of the bottle to release one drop of Opatanol at a time.
- Do not squeeze the bottle, as it is designed to release a drop with a gentle pressure on the base (Figure 2).
- If you are applying drops to both eyes, repeat the above steps for the other eye.
- Screw the cap back on the bottle immediately after use.
If a drop falls outside the eye, try again.
If you use more Opatanol than you should
You can rinse your eyes with warm water. Do not apply more drops until the next application.
If you forget to use Opatanol
Apply a drop as soon as you remember and continue with your regular schedule. However, if it is almost time for your next dose, do not apply the missed dose and continue with your regular schedule. Do not apply a double dose to make up for missed doses.
If you stop using Opatanol
Do not stop using this medicine without consulting your doctor first.
If you have any other questions about using this medicine, ask your doctor or pharmacist.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
The following side effects have been observed with Opatanol:
Common (may affect up to 1 in 10 people)
Eye effects
Eye pain, eye irritation, dry eye, abnormal sensation in the eye, eye discomfort.
General effects
Headache, fatigue, dry nose, bad taste in the mouth.
Uncommon (may affect up to 1 in 100 people)
Eye effects
Blurred vision, decreased or abnormal vision, corneal disorder, inflammation of the eye surface with or without damage to the surface, infection or inflammation of the conjunctiva, eye discharge, sensitivity to light, increased tear production, eye itching, eye redness, eyelid abnormality, itching, redness, swelling, or crust on the eyelid.
General effects
Decreased or abnormal sensation, dizziness, runny nose, dry skin, skin inflammation.
Frequency not known (cannot be estimated from the available data)
Eye effects
Eye swelling, corneal swelling, change in pupil size.
General effects
Difficulty breathing, increased allergic symptoms, facial swelling, numbness, general weakness, nausea, vomiting, sinus infection, redness and itching of the skin.
In very rare cases, some patients with severe damage to the transparent layer on the front of the eye (the cornea) have developed dark spots on the cornea due to calcium accumulation during treatment.
Reporting of side effects
If you experience any side effects, ask your doctor or pharmacist, even if they are side effects not listed in this package leaflet. You can also report them directly through the national reporting system listed in Appendix V. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of Opatanol
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date stated on the bottle and carton after "EXP". The expiry date is the last day of the month stated.
This medicine does not require any special storage conditions.
To avoid infections, you should discard the bottle 4 weeks after first opening and use a new bottle. Write the date of opening on the space provided on the label of each bottle and carton.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines that are no longer needed. This will help protect the environment.
6. Package contents and additional information
Composition of Opatanol
- The active ingredient is olopatadine. Each ml of solution contains 1 mg of olopatadine (as hydrochloride).
- The other ingredients are benzalkonium chloride, sodium chloride, disodium phosphate dodecahydrate (E339), hydrochloric acid (E507) and/or sodium hydroxide (E524) and purified water.
Appearance and package contents
Opatanol is a clear and colorless liquid (a solution) that comes in a bottle containing 5 ml or three plastic bottles of 5 ml with a screw cap.
Not all pack sizes may be marketed.
Marketing authorisation holder
Novartis Europharm Limited
Vista Building
Elm Park, Merrion Road
Dublin 4
Ireland
Manufacturer
Novartis Farmacéutica, S.A.
Gran Via de les Corts Catalanes, 764
08013 Barcelona
Spain
Novartis Manufacturing N.V.
Rijksweg 14
2870 Puurs-Sint-Amands
Belgium
Novartis Pharma GmbH
Roonstraße 25
D-90429 Nuremberg
Germany
Siegfried El Masnou, S.A.
Camil Fabra 58
El Masnou
08320 Barcelona
Spain
Novartis Pharma GmbH
Sophie-Germain-Strasse 10
90443 Nürnberg
Germany
You can obtain more information about this medicine by contacting the local representative of the marketing authorisation holder.
Belgium Novartis Pharma N.V. Tel: +32 2 246 16 11 | Lithuania SIA Novartis Baltics Lietuvos filialas Tel: +370 5 269 16 50 |
Bulgaria Novartis Bulgaria EOOD Tel: +359 2 489 98 28 | Luxembourg Novartis Pharma N.V. Tel: +32 2 246 16 11 |
Czech Republic Novartis s.r.o. Tel: +420 225 775 111 | Hungary Novartis Hungária Kft. Tel: +36 1 457 65 00 |
Denmark Novartis Healthcare A/S Tel: +45 39 16 84 00 | Malta Novartis Pharma Services Inc. Tel: +356 2122 2872 |
Germany Novartis Pharma GmbH Tel: +49 911 273 0 | Netherlands Novartis Pharma B.V. Tel: +31 88 04 52 111 |
Estonia SIA Novartis Baltics Eesti filiaal Tel: +372 66 30 810 | Norway Novartis Norge AS Tel: +47 23 05 20 00 |
Greece Novartis (Hellas) A.E.B.E. Tel: +30 210 281 17 12 | Austria Novartis Pharma GmbH Tel: +43 1 86 6570 |
Spain Novartis Farmacéutica, S.A. Tel: +34 93 306 42 00 | Poland Novartis Poland Sp. z o.o. Tel: +48 22 375 4888 |
France Novartis Pharma S.A.S. Tel: +33 1 55 47 66 00 | Portugal Novartis Farma - Produtos Farmacêuticos, S.A. Tel: +351 21 000 8600 |
Croatia Novartis Hrvatska d.o.o. Tel: +385 1 6274 220 | Romania Novartis Pharma Services Romania SRL Tel: +40 21 31299 01 |
Ireland Novartis Ireland Limited Tel: +353 1 260 12 55 | Slovenia Novartis Pharma Services Inc. Tel: +386 1 300 75 50 |
Iceland Vistor hf. Tel: +354 535 7000 | Slovakia Novartis Slovakia s.r.o. Tel: +421 2 5542 5439 |
Italy Novartis Farma S.p.A. Tel: +39 02 96 54 1 | Finland Novartis Finland Oy Tel: +358 (0)10 6133 200 |
Cyprus Novartis Pharma Services Inc. Tel: +357 22 690 690 | Sweden Novartis Sverige AB Tel: +46 8 732 32 00 |
Latvia SIA Novartis Baltics Tel: +371 67 887 070 |
Date of last revision of this package leaflet:
Other sources of information
Detailed information about this medicine is available on the European Medicines Agency website: http://www.ema.europa.eu
- Country of registration
- Average pharmacy price7.74 EUR
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to OPATANOL 1 mg/ml EYE DROPS SOLUTIONDosage form: EYEDROP, 1 mg/mlActive substance: olopatadineManufacturer: Tiedra Farmaceutica S.L.Prescription requiredDosage form: EYEDROP, 1 mg/mlActive substance: olopatadineManufacturer: Qualix Pharma S.L.Prescription requiredDosage form: EYE DROP, 1mg/ml mg/mlActive substance: olopatadineManufacturer: Juta Pharma GmbhPrescription required
Online doctors for OPATANOL 1 mg/ml EYE DROPS SOLUTION
Discuss questions about OPATANOL 1 mg/ml EYE DROPS SOLUTION, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions















