OLOPATADINE ABAMED 1 mg/ml EYE DROPS SOLUTION

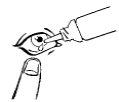
How to use OLOPATADINE ABAMED 1 mg/ml EYE DROPS SOLUTION
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the Patient
Olopatadina Abamed1 mg/ml eye drops solution
Olopatadina
L
- Keep this leaflet, as you may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you experience any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. See section 4.
C
- What Olopatadina Abamed is and what it is used for
- What you need to know before you use Olopatadina Abamed
- How to use Olopatadina Abamed
- Possible side effects
- Storage of Olopatadina Abamed
Contents of the pack and further information
1. What Olopatadina Abamed is and what it is used for
Olopatadina Abamed belongs to a group of medicines used to treat allergic conditions in the eyes. It works by reducing the intensity of the allergic reaction.
Olopatadina Abamed is indicated for the treatment of the signs and symptoms of seasonal allergic conjunctivitis.
In allergic conjunctivitis, certain elements (called allergens) such as pollen, house dust, or animal hair can cause allergic reactions that lead to itching and redness, as well as inflammation of the surface of your eyes.
2. What you need to know before you use Olopatadina Abamed
N
- If you are allergic (hypersensitive) to olopatadine or any of the other ingredients of this medicine (listed in section 6).
- If you are breastfeeding.
Warnings and precautions
Consult your doctor or pharmacist before starting to use Olopatadina Abamed.
If you wear contact lenses, you should remove them before using Olopatadina Abamed.
Children
Do not give this medicine to children under 3 years of age, as its safety and efficacy have not been established.
Using Olopatadina Abamed with other medicines
Tell your doctor or pharmacist if you are using, have recently used, or might use any other medicines.
If you are using another eye drop or ointment, wait at least 5 minutes between applying each medicine. Ointments should be applied last.
Pregnancy, breastfeeding, and fertility
If you are pregnant, think you may be pregnant, or are planning to become pregnant, consult your doctor or pharmacist before using this medicine.
Do not use Olopatadina Abamed if you are breastfeeding. Consult your doctor before using this medicine.
Driving and using machines
Immediately after applying Olopatadina Abamed, you may notice that your vision is blurred. Do not drive or use machines until this effect has disappeared.
Olopatadina Abamed contains benzalkonium chloride, which may:
- cause eye irritation
- discolor soft contact lenses
It is recommended:
- to avoid contact with soft contact lenses
remove contact lenses before applying the eye drops and wait at least 15 minutes before putting them back in.
3. How to use Olopatadina Abamed
Follow exactly the instructions of administration of this medicine given by your doctor or pharmacist. If you are in doubt, consult your doctor or pharmacist again. Remember to use your medicine.
The recommended dose is one drop in one eye or in both eyes, twice a day: in the morning and in the evening.
Use this amount unless your doctor has given you other instructions. Olopatadina Abamed should only be applied to both eyes if your doctor has told you to do so. Continue the treatment for the period of time indicated by your doctor.
Remember that this medicine should only be applied to the eyes.
Instructions for the proper use of Olopatadina Abamed:
To facilitate the application of the eye drops, the first few times it may be helpful to sit in front of a mirror so that you can see what you are doing.
| |
| |
| |
| |
| |
| |
|
|
|
|
|
Drawing 1 | Drawing 2 | Drawing 3 | Drawing 4 |
If a drop falls outside the eye, try again.
S
If you have accidentally administered too much Olopatadina Abamed in your eyes, you can remove it by rinsing your eyes with lukewarm water. In this case, do not administer more drops until the next application.
In case of accidental ingestion, consult your doctor, the emergency department of the nearest hospital, or call the Toxicology Information Service (91) 562 04 20, indicating the medicine and the amount administered.
S
Apply one drop of eye drops as soon as you remember and administer the next dose at your usual time.
However, if it is almost time for the next dose, do not apply the missed dose and continue with the next dose. Do not apply a double dose to make up for the missed dose.
S
Do not stop using this medicine without consulting your doctor first.
If you have any further questions about the use of this medicine, ask your doctor or pharmacist.
4. Possible side effects
Like all medicines, Olopatadina Abamed can cause side effects, although not everybody gets them.
The side effects that have been seen during the use of this eye drop are:
Common(may affect up to 1 in 10 people):
- Eye effects: eye pain, eye irritation, dry eye, abnormal sensation in the eye, eye discomfort.
- General effects: headache, fatigue, dry nose, bad taste in the mouth.
Uncommon(may affect up to 1 in 100 people):
- Eye effects: blurred, decreased, or abnormal vision, corneal disorder, inflammation of the eye surface with or without damage to the surface, infection or inflammation of the conjunctiva, eye discharge, sensitivity to light, increased tear production, eye itching, eye redness, eyelid abnormality, itching, redness, swelling, or crust on the eyelid.
- General effects: decreased or abnormal perception, dizziness, runny nose, dry skin, skin inflammation.
F
- Eye effects: eye swelling, corneal swelling, change in pupil size.
- General effects: difficulty breathing, increased allergic symptoms, facial swelling, numbness, general weakness, nausea, vomiting, sinus infection, redness, and itching of the skin.
In very rare cases, some patients with severe damage to the transparent layer on the front of the eye (the cornea) have developed dark spots on the cornea due to calcium accumulation during treatment.
Reporting of side effects
If you experience any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. You can also report side effects directly through the Spanish Pharmacovigilance System for Human Use Medicines: www.notificaram.es. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of Olopatadina Abamed
Keep this medicine out of the sight and reach of children.
No special storage conditions are required.
To avoid infections, you should discard the bottle four weeks after first opening it and use a new bottle. Note the date of opening on the space provided on the label of each bottle and on the carton.
Do not use this medicine after the expiry date which is stated on the bottle (after EXP.). The expiry date is the last day of the month shown.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. These measures will help protect the environment.
6. Contents of the pack and further information
Composition of Olopatadina Abamed
- The active substance is olopatadine. Each milliliter of eye drops contains 1 mg of olopatadine (as hydrochloride).
- The other ingredients are benzalkonium chloride, sodium chloride, disodium hydrogen phosphate dodecahydrate (E-339), hydrochloric acid (E-507), and/or sodium hydroxide (E-524) and water for injection.
Appearance of the product and contents of the pack
Olopatadina Abamed is a colorless, transparent solution. Each bottle contains 5 ml of eye drops.
Marketing authorization holder:
Qualix Pharma, S.L.
c/ Botánica, 137-139
08908 L’Hospitalet de Llobregat
(Barcelona)
Spain
Manufacturer:
Labiana Pharmaceuticals, S.L.U.
c/ Casanova 27-31
08757 Corbera de Llobregat
(Barcelona)
Spain
Date of the last revision of this leaflet:November 2018
Detailed and up-to-date information on this medicine is available on the website of the Spanish Agency for Medicines and Health Products (AEMPS) http://www.aemps.gob.es/
- Country of registration
- Average pharmacy price7.74 EUR
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to OLOPATADINE ABAMED 1 mg/ml EYE DROPS SOLUTIONDosage form: EYEDROP, 1 mg/mlActive substance: olopatadineManufacturer: Tiedra Farmaceutica S.L.Prescription requiredDosage form: EYE DROP, 1mg/ml mg/mlActive substance: olopatadineManufacturer: Juta Pharma GmbhPrescription requiredDosage form: EYE DROP, 1 mg/mlActive substance: olopatadineManufacturer: Novartis Europharm LimitedPrescription required
Online doctors for OLOPATADINE ABAMED 1 mg/ml EYE DROPS SOLUTION
Discuss questions about OLOPATADINE ABAMED 1 mg/ml EYE DROPS SOLUTION, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions