
VIVIDRIN 0.5 mg/ml EYE DROPS IN SOLUTION IN SINGLE-DOSE CONTAINERS

How to use VIVIDRIN 0.5 mg/ml EYE DROPS IN SOLUTION IN SINGLE-DOSE CONTAINERS
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the User
Vividrin 0.5 mg/ml eye drops, solution in single-dose container
azelastine hydrochloride
Read this package leaflet carefully before you start using this medicine, because it contains important information for you.
Follow exactly the administration instructions of the medicine contained in this package leaflet or as indicated by your doctor, pharmacist, or nurse.
- Keep this package leaflet, you may need to read it again.
- If you need advice or more information, consult your doctor or pharmacist.
- If you experience side effects, consult your doctor or pharmacist, even if they are not listed in this package leaflet. See section 4.
- If after 48 hours you do not feel better or worsen, consult your doctor.
Contents of the package leaflet
- What is Vividrin and what is it used for
- What you need to know before you start using Vividrin
- How to use Vividrin
- Possible side effects
- Storage of Vividrin
- Contents of the pack and further information
1. What is Vividrin and what is it used for
This medicine contains azelastine hydrochloride, which belongs to a group of known antihistamines. Antihistamines prevent the effects of substances such as histamine that are produced by the body as part of an allergic reaction. Azelastine has been shown to reduce ocular inflammation.
This medicine is used in the following situations:
- Treatment and prevention of ocular disorders caused by hay fever (seasonal allergic conjunctivitis) in adults and children from 4 years of age.
- Treatment of ocular disorders caused by allergy to dust mites or animal hair (perennial allergic conjunctivitis) in adults and children from 12 years of age.
This medicine is notsuitable for the treatment of eye infections.
2. What you need to know before you start using Vividrin
Do not use Vividrin:
If you are allergic to azelastine hydrochloride or any of the other components of this medicine (listed in section 6).
Warnings and precautions
Consult your doctor or pharmacist before starting to use this medicine:
- If you are not sure that your eye symptoms are caused by an allergy. In particular, if they only affect one eye, if your vision has worsened, or if you have eye pain and no nasal symptoms, it may be an infection rather than an allergy.
- If symptoms worsen or last more than 48 hours without noticeable improvement despite using this medicine.
- If you wear contact lenses.
Children and adolescents
This medicine should not be used in children under 4 years of age for the treatment and prevention of ocular disorders related to hay fever (seasonal allergic conjunctivitis).
This medicine should not be used in children under 12 years of age for the treatment of ocular disorders caused by allergy to dust mites or animal hair (perennial allergic conjunctivitis).
Other medicines and Vividrin
Tell your doctor or pharmacist if you are using, have recently used, or might use any other medicine.
It is not known if this medicine can be affected by other medicines.
Pregnancy, breastfeeding, and fertility
If you are pregnant or breastfeeding, think you may be pregnant, or plan to become pregnant, consult your doctor before using this medicine.
Driving and using machines
You may notice blurred vision for a short period after applying this medicine. If this happens, wait until your vision returns before driving or using machinery.
3. How to use Vividrin
Follow exactly the administration instructions of this medicine contained in this package leaflet or as indicated by your doctor or pharmacist. If in doubt, consult your doctor or pharmacist again. Remember: this medicine should only be applied to the eyes.
The recommended dose is:
Ocular disorders caused by hay fever (seasonal allergic conjunctivitis)
Use in adults and children from 4 years of age.
The usual dose is one drop in each eye, morning and night.
If you anticipate exposure to pollen, you can take the usual dose of this medicine as a preventive measure before going outside.
Ocular disorders caused by allergy (perennial allergic conjunctivitis)
Use in adults and children from 12 years of age.
The usual dose is one drop in each eye, morning and night.
If your symptoms are severe, your doctor may increase the dose to one drop in each eye up to four times a day.
Relief from allergic conjunctivitis symptoms should be noticed within 15-30 minutes.
Method of administration (application of the eye drops)
To facilitate the correct application of the eye drops, it may be helpful to sit in front of a mirror so you can see what you are doing.
- Wash your hands.
- Gently clean the contour of your eyes with a cloth to remove any moisture.
- Carefully separate a single-dose container from the top of the strip (Fig. 1) and open it. Twist and separate the top of the single-dose unit (without pulling it) (Fig. 2).
- Tilt your head back.
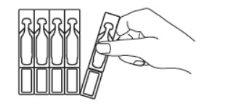
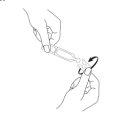
Fig. 1 Fig. 2
- Gently pull the lower eyelid down.
- Place one drop carefully inside the middle area of the lower eyelid. Make sure the container does not touch the eye to avoid contamination (Fig. 3).
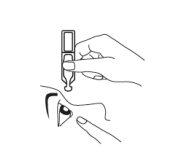
Fig. 3.
- Release the lower eyelid and gently press the inner corner of the eye against the bridge of the nose. While the finger presses the nose, blink slowly a few times to spread the drop across the entire surface of the eye.
- Wipe away any excess medicine with a cloth.
- Repeat the procedure with the other eye.
Duration of treatment
If possible, use this medicine regularly until symptoms have disappeared. If you stop using this medicine, it is likely that your symptoms will recur.
Do not use this medicine for more than 6 weeks.
If you use more Vividrin than you should:
If you accidentally administer too much Vividrin in the eye, it is likely that you will not have any problems. If you have any doubts, consult your doctor. In case of accidental ingestion of this medicine, contact your doctor or go to the emergency department of the nearest hospital immediately.
If you forget to use Vividrin
Use your eye drops as soon as you remember. Apply the next dose at the usual time. Do not apply a double dose to make up for the forgotten dose.
If you stop using Vividrin
If you have any other doubts about using this medicine, ask your doctor or pharmacist.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Common side effects (may affect up to 1 in 10 people):
Mild irritation (stinging, itching, tearing) in the eyes after using this medicine. These effects should not last long.
Uncommon side effects (may affect up to 1 in 100 people):
Bitter taste in the mouth. This effect should disappear quickly, especially if you take a drink.
Rare side effects (may affect up to 1 in 10,000 people):
Allergic reaction (such as rash and itching).
Reporting side effects
If you experience side effects, consult your doctor or pharmacist, even if they are not listed in this package leaflet. You can also report them directly through the Spanish Medicines Agency's website: https://www.notificaram.es. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of Vividrin
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiration date stated on the carton and on the container, after "EXP".
Use the single-dose container only once.
Medicines should not be disposed of via wastewater or household waste. Return the containers and medicines you no longer need to the pharmacy. If in doubt, ask your pharmacist how to dispose of the containers and medicines you no longer need. This will help protect the environment.
Do not store above 25°C.
6. Contents of the pack and further information
Composition of Vividrin
- The active substance is azelastine hydrochloride.
Each milliliter contains 0.5 mg of azelastine hydrochloride.
Each drop contains 0.018 mg of azelastine hydrochloride.
- The other ingredients are:
Sorbitol 70% solution (non-crystallizing), hypromellose, disodium edetate, sodium hydroxide, and water for injections.
Appearance of Vividrin and contents of the container
This medicine is a colorless and transparent solution presented in a transparent LDPE single-dose container with a volume of 0.6 ml.
Each carton contains 10, 20, 30, or 60 single-dose containers.
Not all pack sizes may be marketed.
Marketing authorization holder and manufacturer
Marketing authorization holder:
BAUSCH + LOMB IRELAND LIMITED
3013 Lake Drive
Citywest Business Campus
Dublin 24, D24PPT3
Ireland
Local representative in Spain
Bausch & Lomb S.A.
Avda. Valdelaparra, nº 4
28108 Alcobendas
Madrid, Spain.
Tel: 91 – 657 63 00
Manufacturer:
DR. GERHARD MANN. CHEM.
Brunsbütteler Damm 165/173,
13581 Berlin.
Germany
This medicine is authorized in the Member States of the European Economic Area under the following names:
DE:Vividrin Azelastin EDO 0,5 mg/ml Augentropfen, Lösung im Einzeldosisbehältnis |
NL:Azelergo 0,5 mg/ml oogdruppels, oplossing
AT:Vividrin Azelastin EDO 0,5 mg/ml Augentropfen, Lösung im Einzeldosisbehältnis IT:Monodrin occhi SI:Alezaxin 0,5 mg/ml kapljice za oko, raztopina v enoodmernem vsebniku |
BE:Azelergo 0,5 mg/ml collyre en solution en récipient unidose |
LU:Azelergo 0,5 mg/ml collyre en solution en récipient unidose
PT:Vivilin
ES:Vividrin 0,5 mg/ml colirio en solución en envase unidosis
EE:Vividrin
This package leaflet was last revised inMay 2021
Detailed information on this medicine is available on the website of the Spanish Agency for Medicines and Health Products (AEMPS) (http://www.aemps.gob.es/).
- Country of registration
- Average pharmacy price17.61 EUR
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to VIVIDRIN 0.5 mg/ml EYE DROPS IN SOLUTION IN SINGLE-DOSE CONTAINERSDosage form: EYEDROP, 0.5 mg/mlActive substance: azelastineManufacturer: Cooper Consumer Health B.V.Prescription requiredDosage form: EYEDROP, 0.5 mg/mlActive substance: azelastineManufacturer: Qualix Pharma S.L.Prescription requiredDosage form: EYE DROP, 0.5 mg/mlActive substance: azelastineManufacturer: Mabo Farma S.A.Prescription required
Online doctors for VIVIDRIN 0.5 mg/ml EYE DROPS IN SOLUTION IN SINGLE-DOSE CONTAINERS
Discuss questions about VIVIDRIN 0.5 mg/ml EYE DROPS IN SOLUTION IN SINGLE-DOSE CONTAINERS, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions















