
How to use Mirgi
Leaflet accompanying the packaging: information for the user
Mirgi, (0.12 mg + 0.015 mg)/24 h, intrauterine therapeutic system
Etonogestrel + Ethinylestradiol
Important information about combined hormonal contraceptives
- If used correctly, they are one of the most reliable, reversible methods of contraception.
- They slightly increase the risk of blood clots in veins and arteries, especially in the first year of use or after resuming use after a break of 4 weeks or more.
- Caution should be exercised and a doctor should be consulted if the patient suspects that symptoms of blood clots have occurred (see section 2 "Blood clots").
The leaflet should be carefully read before using the medicine, as it contains important information for the patient.
- The leaflet should be kept so that it can be re-read if necessary.
- In case of any doubts, a doctor or pharmacist should be consulted.
- This medicine has been prescribed to a specific person. It should not be given to others. The medicine may harm another person.
- If the patient experiences any side effects, including any side effects not listed in this leaflet, they should tell their doctor or pharmacist. See section 4.
Table of contents of the leaflet
1. What Mirgi is and what it is used for
2. Important information before using Mirgi
- 2.1 When not to use Mirgi
- 2.2 Warnings and precautions Blood clots Cancer
- 2.3 Children and adolescents
- 2.4 Mirgi and other medicines Diagnostic tests
- 2.5 Pregnancy and breastfeeding
- 2.6 Driving and using machines
3. How to use Mirgi
- 3.1 Insertion and removal of Mirgi
- 3.2 Three weeks of use, one week of break
- 3.3 When to insert the first Mirgi intrauterine therapeutic system
- 3.4 What to do… Procedure in case of accidental expulsion of the system from the vaginaProcedure when the system has been outside the vagina for some timeProcedure in case of damage to the intrauterine therapeutic systemProcedure when more than one intrauterine therapeutic system has been insertedProcedure when, after a one-week break, the patient has forgotten to insert a new intrauterine therapeutic systemProcedure when the patient has forgotten to remove the intrauterine therapeutic system on timeProcedure in case of absence of bleedingProcedure in case of unexpected bleedingProcedure in case of change in the day of bleedingProcedure in case of desire to delay the onset of bleeding
- 3.5 Procedure when the patient wants to stop using Mirgi
4. Possible side effects
5. How to store Mirgi
6. Contents of the packaging and other information
What Mirgi contains
How Mirgi looks and what the packaging contains Responsible entity and manufacturer
1. What Mirgi is and what it is used for
Mirgi is a contraceptive in the form of an intrauterine therapeutic system, preventing pregnancy. Each intrauterine therapeutic system contains a small amount of two female sex hormones - etonogestrel and ethinylestradiol. These hormones are slowly released from the system into the bloodstream. Due to the small dose of hormones released, Mirgi is classified as a low-dose hormonal contraceptive. Since Mirgi releases two different hormones, it is also a combined hormonal contraceptive.
Mirgi works like a combined oral contraceptive pill, but unlike a pill that needs to be taken every day, Mirgi is used for 3 weeks in a row. Mirgi releases two female sex hormones that inhibit the release of egg cells from the ovaries. Since egg cells are not released, the patient cannot become pregnant.
2. Important information before using Mirgi
General notes
Before starting to use Mirgi, you should read the information about blood clots (thrombosis) in section 2. It is especially important to read about the symptoms of blood clots - see section 2 "Blood clots".
Mirgi, like other hormonal contraceptives, does not protect against HIV (AIDS) or other sexually transmitted diseases.
2.1 When not to use Mirgi
Mirgi should not be used if the patient has any of the following conditions. If the patient has any of the following conditions, they must inform their doctor. The doctor will discuss with the patient which other contraceptive method will be more suitable.
- if the patient currently has (or has ever had) a blood clot in the veins of the legs (deep vein thrombosis), in the lungs (pulmonary embolism), or in other organs,
- if the patient knows they have a blood clotting disorder - such as protein C deficiency, protein S deficiency, antithrombin III deficiency, factor V Leiden, or antiphospholipid antibodies,
- if the patient needs to have surgery or will be immobilized for a long time (see section "Blood clots"),
- if the patient has had a heart attack or stroke,
- if the patient has (or has had in the past) angina pectoris (a disease that causes severe chest pain, which can be the first symptom of a heart attack) or a transient ischemic attack (temporary stroke symptoms),
- if the patient has any of the following diseases, which may increase the risk of a blood clot in an artery:
- severe diabetes with blood vessel damage
- very high blood pressure
- very high levels of fats in the blood (cholesterol or triglycerides)
- hyperhomocysteinemia
- if the patient has (or has had in the past) a type of migraine called "migraine with aura",
- if the patient has (or has had in the past) pancreatitis associated with high levels of fats in the blood,
- if the patient has (or has had in the past) severe liver disease, and liver function has not returned to normal,
- if the patient has (or has had in the past) a benign or malignant liver tumor,
- if the patient has (or has had in the past) breast cancer or genital organ cancer, or if such cancers are suspected,
- if the patient has unexplained vaginal bleeding,
- if the patient is allergic to ethinylestradiol or etonogestrel or any of the other ingredients of this medicine (listed in section 6).
If any of the above conditions occur for the first time while using Mirgi, the system should be removed from the vagina and a doctor should be consulted immediately. If the patient experiences any of the above conditions, they should stop using Mirgi and use a non-hormonal contraceptive method until the condition is resolved.
2.2 Warnings and precautions
Before starting to use Mirgi, the patient should discuss it with their doctor or pharmacist.
The patient should contact their doctor immediately:
- if they notice any symptoms of a blood clot, which may indicate that they have a blood clot in their leg (deep vein thrombosis), a blood clot in their lungs (pulmonary embolism), a heart attack, or a stroke (see below "Blood clots (thrombosis)").
The patient should tell their doctor if they have any of the following conditions.
If these conditions occur or worsen while using Mirgi, the patient should also tell their doctor.
- if breast cancer is present or has occurred in close relatives;
- if the patient has epilepsy (see section 2.4 "Mirgi and other medicines");
- if the patient has liver disease (e.g., jaundice) or gallbladder disease (e.g., gallstones);
- if the patient has Crohn's disease or ulcerative colitis (chronic inflammatory bowel diseases);
- if the patient has systemic lupus erythematosus (a disease that affects the body's natural defense system);
- if the patient has hemolytic uremic syndrome (a blood clotting disorder that causes kidney failure);
- if the patient has sickle cell anemia (a genetic disorder that affects red blood cells);
- if the patient has high levels of fats in the blood (hypertriglyceridemia) or a family history of this condition. Hypertriglyceridemia is associated with an increased risk of developing pancreatitis (inflammation of the pancreas);
- if the patient needs to have surgery or will be immobilized for a long time (see section "Blood clots");
- if the patient has recently given birth, as they are at increased risk of blood clots. The patient should consult their doctor for information on when to start using Mirgi after giving birth;
- if the patient has superficial thrombophlebitis (inflammation of the veins just under the skin);
- if the patient has varicose veins;
- if the patient has diseases that first occurred or worsened during pregnancy or previous use of sex hormones (e.g., hearing loss, porphyria [a blood disorder], herpes during pregnancy [a blistering skin rash during pregnancy], Sydenham's chorea [a neurological disorder that causes involuntary, jerky movements of the body]);
- if the patient experiences symptoms of angioedema, such as swelling of the face, tongue, and/or throat, and/or difficulty swallowing or hives that may cause difficulty breathing, they should contact their doctor immediately. Products containing estrogens may cause or worsen symptoms of hereditary or acquired angioedema.
- if the patient has chloasma (melasma) now or in the past (brown patches, so-called "pregnancy patches", especially on the face). If these occur, the patient should avoid excessive sunlight and ultraviolet radiation;
- if the patient has conditions that make it difficult to use Mirgi - such as frequent constipation, uterine prolapse, or pain during intercourse;
- if the patient experiences sudden, frequent need to urinate with a burning sensation and/or pain, and/or if they are unable to locate the intrauterine therapeutic system in the vagina. These symptoms may indicate that the Mirgi intrauterine therapeutic system has been accidentally inserted into the bladder.
BLOOD CLOTS
The use of combined hormonal contraceptives, such as the Mirgi intrauterine therapeutic system, is associated with an increased risk of blood clots, compared to not using them. In rare cases, a blood clot can block a blood vessel and cause serious complications.
Blood clots can occur:
- in veins (also known as "venous thromboembolism" or "deep vein thrombosis");
- in arteries (also known as "arterial thromboembolism").
Not everyone recovers fully from a blood clot. In rare cases, the effects of a blood clot can be long-lasting or, very rarely, fatal.
It should be remembered that the overall risk of serious blood clots caused by Mirgi is small.
HOW TO RECOGNIZE THE FORMATION OF BLOOD CLOTS
The patient should contact their doctor immediately if they notice any of the following symptoms.
Is the patient experiencing any of these symptoms?
Why is the patient likely suffering?
- swelling of the leg or swelling along a vein in the leg or foot, especially if it is accompanied by:
- pain or tenderness in the leg, which may only be felt when standing or walking
- increased temperature in the affected leg
- change in the color of the leg skin, such as pallor, redness, or cyanosis Deep vein thrombosis
Pulmonary embolism
- sudden unexplained shortness of breath or rapid breathing;
- sudden unexplained cough, which may be accompanied by coughing up blood;
- sharp chest pain, which may worsen with deep breathing;
- severe dizziness or fainting;
- rapid or irregular heartbeat;
- severe abdominal pain.
If the patient is unsure, they should contact their doctor, as some of these symptoms, such as coughing or shortness of breath, may be mistaken for less serious conditions, such as a respiratory infection (e.g., a cold).
Symptoms usually occur in one eye:
- sudden loss of vision or
- painless vision disturbances, which may progress to loss of vision Retinal vein thrombosis (blood clot in the eye)
Heart attack
- chest pain, discomfort, pressure, heaviness;
- a feeling of squeezing or fullness in the chest, arm, or below the breastbone;
- a feeling of fullness, indigestion, or choking;
- discomfort in the upper body radiating to the back, jaw, throat, arm, and stomach;
- sweating, nausea, vomiting, or fainting;
- extreme weakness, anxiety, or shortness of breath;
- rapid or irregular heartbeat.
| Stroke |
| Blood clots blocking other blood vessels |
BLOOD CLOTS IN VEINS
What can happen if blood clots form in veins?
- The use of combined hormonal contraceptives is associated with an increased risk of blood clots in veins (venous thromboembolism). Although these side effects are rare, they can occur.
- If blood clots form in the veins of the leg or foot, it can lead to the development of deep vein thrombosis.
- If a blood clot moves from the leg and settles in the lungs, it can cause a pulmonary embolism.
- In very rare cases, a blood clot can form in another organ, such as the eye (retinal vein thrombosis).
When is the risk of blood clots in veins highest?
The risk of forming blood clots in veins is highest during the first year of using combined hormonal contraceptives for the first time. The risk may also be higher when resuming the use of combined hormonal contraceptives (the same or a different medicine) after a break of 4 weeks or more.
After the first year, the risk decreases, but it is always higher compared to not using combined hormonal contraceptives.
If the patient stops using Mirgi, the risk of blood clots returns to normal within a few weeks.
What factors increase the risk of blood clots in veins?
The risk of blood clots associated with Mirgi is small, but some factors can increase this risk.
The risk is higher:
- if the patient is severely overweight (body mass index (BMI) over 30 kg/m2);
- if someone in the patient's close family has had blood clots in the legs, lungs, or other organs at a young age (e.g., under 50 years old). In this case, the patient may have a hereditary blood clotting disorder;
- if the patient needs to have surgery or will be immobilized for a long time (see section "Blood clots");
- with age (especially over 35 years);
- if the patient has recently given birth.
The risk of blood clots increases with the number of risk factors present in the patient.
Air travel (>4 hours) may temporarily increase the risk of blood clots, especially if the patient has another risk factor.
It is essential to tell the doctor if any of these risk factors are present, even if the patient is unsure. The doctor may decide to stop the use of Mirgi.
The patient should inform their doctor if any of the above conditions change while using Mirgi, e.g., if someone in their close family is diagnosed with a blood clot without a known cause or if the patient gains significant weight.
BLOOD CLOTS IN ARTERIES
What can happen if blood clots form in arteries?
Similar to blood clots in veins, blood clots in arteries can cause serious complications, such as a heart attack or stroke.
What factors increase the risk of blood clots in arteries?
It is essential to note that the risk of heart attack or stroke associated with Mirgi is very small, but it may increase:
- with age (over approximately 35 years);
- if the patient smokes. During the use of a hormonal contraceptive like Mirgi, it is recommended to quit smoking. If the patient is unable to quit smoking and is over 35 years old, the doctor may recommend using a different type of contraception;
- if the patient is overweight;
- if the patient has high blood pressure;
- if someone in the patient's close family has had a heart attack or stroke at a young age (under 50 years old). In this case, the patient may also be at increased risk of having a heart attack or stroke;
- if the patient or someone in their close family has high levels of fats in the blood (cholesterol or triglycerides);
- if the patient has migraines, especially migraines with aura;
- if the patient has heart disease (valve damage, arrhythmia called atrial fibrillation);
- if the patient has diabetes.
If the patient has more than one of the above conditions or if any of them are severe, the risk of blood clots may be further increased.
The patient should inform their doctor if any of the above conditions change while using Mirgi, e.g., if they start smoking, if someone in their close family is diagnosed with a blood clot without a known cause, or if they gain significant weight.
CANCER
The following information is based on studies using combined oral contraceptives and may also apply to Mirgi. Information on the vaginal use of hormonal contraceptives (as with Mirgi) is not available.
Among women using combined contraceptives, there is a slightly increased risk of breast cancer, although it is not known if this is caused by the medicines. It is possible that women using combined contraceptives are more likely to have their breasts examined, which may lead to more breast cancers being detected.
The increased risk of breast cancer disappears gradually after stopping the use of combined contraceptives.
Regular breast examination is very important. If a lump is found, the patient should contact their doctor. The patient should also inform their doctor if breast cancer has occurred or is occurring in close relatives (see section 2.2 "Warnings and precautions").
In rare cases, women using combined contraceptives have developed benign liver tumors, and very rarely, malignant liver tumors. If the patient experiences unusual, severe abdominal pain, they should contact their doctor.
There are reports that women using combined contraceptives are less likely to develop endometrial cancer (cancer of the lining of the uterus) and ovarian cancer. It is possible that this also applies to Mirgi, but this has not been confirmed yet.
PSYCHIATRIC DISORDERS
Some women using hormonal contraceptives, including Mirgi, have reported depression or low mood. Depression can be severe and sometimes lead to suicidal thoughts.
If mood changes and symptoms of depression occur, the patient should contact their doctor as soon as possible for further medical advice.
2.3 Children and adolescents
The safety and efficacy of Mirgi have not been studied in adolescents under the age of 18.
2.4 Mirgi and other medicines
The patient should tell their doctor or pharmacist about all medicines they are currently using or have recently used, as well as any medicines they plan to use. The patient should also inform their doctor of another specialty or dentist prescribing other medicines (or pharmacist) about the use of Mirgi.
Some medicines:
- may affect the level of Mirgi in the blood;
- may reduce its contraceptive effectiveness;
- may cause unexpected bleeding.
This applies to medicines used to treat:
- epilepsy (e.g., primidone, phenytoin, barbiturates, carbamazepine, oxcarbazepine, topiramate, felbamate);
- tuberculosis (e.g., rifampicin);
- HIV infection (e.g., ritonavir, nelfinavir, nevirapine, efavirenz);
- hepatitis C virus infection (e.g., boceprevir, telaprevir);
- other infectious diseases (e.g., griseofulvin);
- high blood pressure in the blood vessels of the lungs (bosentan);
- depressive moods (St. John's Wort (Hypericum perforatum)).
If the patient is taking medicines or herbal remedies that may reduce the effectiveness of Mirgi, they should also use a mechanical contraceptive method. Due to the fact that the effect of another medicine on Mirgi may persist for up to 28 days after stopping the medicine, it is necessary to use additional mechanical contraception during this time.
Note: Mirgi should not be used with a diaphragm, cervical cap, or female condom.
Mirgi may affect the action of other medicines, such as:
- cyclosporine
- the antiepileptic drug lamotrigine (this may lead to an increased frequency of seizures)
If the patient has a hepatitis C virus infection and is taking medicines containing ombitasvir, paritaprevir, ritonavir, dasabuvir, glecaprevir with pibrentasvir, or sofosbuvir with velpatasvir and voxilaprevir, they should not use the Mirgi intrauterine therapeutic system, as these medicines may cause an increase in liver function test results in the blood (increase in liver enzyme ALT activity).
Before starting these medicines, the doctor will prescribe a different type of contraceptive.
The use of the Mirgi intrauterine therapeutic system can be resumed about 2 weeks after the end of this treatment. See section 2.1 "When not to use Mirgi".
Before taking any medicine, the patient should consult their doctor or pharmacist.
During the use of Mirgi, tampons can be used at the same time. The patient should insert Mirgi before inserting a tampon. The patient should be careful when removing the tampon to avoid accidentally removing Mirgi as well. If Mirgi is expelled, it can be rinsed with cold or lukewarm water and reinserted as soon as possible.
Damage to the intrauterine therapeutic system has occurred during the use of vaginal products, such as moisturizers or treatments for infections (see section 3.4 "What to do... Procedure when more than one intrauterine therapeutic system has been inserted").
The use of spermicides or vaginal antifungal medicines does not reduce the contraceptive effectiveness of Mirgi.
DIAGNOSTIC TESTS
If laboratory tests of blood or urine are performed, the patient should inform the persons performing the tests about the use of Mirgi, as the use of the intrauterine therapeutic system may affect the results of some laboratory tests.
2.5 Pregnancy and breastfeeding
Mirgi should not be used during pregnancy or if pregnancy is suspected. If the patient becomes pregnant while using Mirgi, the intrauterine therapeutic system should be removed and a doctor should be consulted.
If the patient wants to stop using Mirgi because they want to become pregnant, they should read the information in section 3.5 "Procedure when the patient wants to stop using Mirgi".
The use of Mirgi is not recommended during breastfeeding. If the patient wants to use Mirgi during breastfeeding, they should consult their doctor first.
2.6 Driving and using machines
Mirgi does not affect the ability to drive or use machines.
3. How to use Mirgi
Mirgi can be inserted and removed by the patient themselves. The doctor will instruct the patient on when to start using Mirgi. The intrauterine therapeutic system should be inserted on the appropriate day of the cycle (see section 3.3 "When to insert the first Mirgi intrauterine therapeutic system") and left in place for 3 weeks in a row.
The patient should regularly check if the Mirgi intrauterine therapeutic system is in place in the vagina (e.g., before and after intercourse) to ensure contraceptive protection.
After 3 weeks, the system should be removed and a 1-week break should be taken. Usually, withdrawal bleeding occurs during this break.
Certain mechanical contraceptive methods for women, such as a diaphragm, cervical cap, or female condom, should not be used during the use of Mirgi.
These mechanical contraceptive methods should not be used as an additional contraceptive method, as Mirgi may make it difficult to properly insert and position the diaphragm, cervical cap, or female condom.
However, a male condom can be used as an additional mechanical contraceptive method.
3.1 Insertion and removal of Mirgi
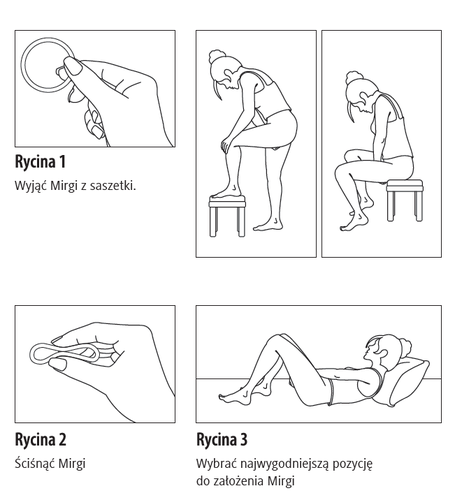
- 1. Before insertion, the patient should check the expiration date (see section 5 "How to store Mirgi").
- 2. Before insertion or removal, the patient should wash their hands.
- 3. The patient should choose the most comfortable position for insertion, such as standing with one leg raised, squatting, or lying down.
- 4. The patient should remove Mirgi from the packaging.
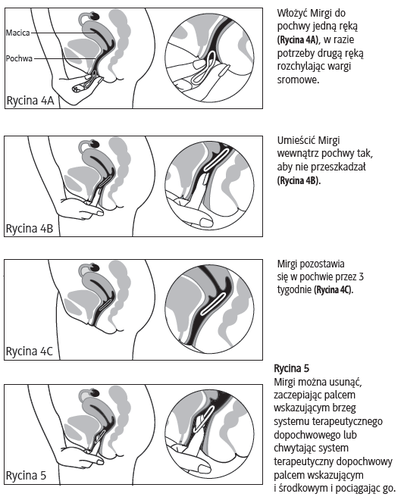
- 5. Holding the system between the thumb and index finger, the patient should squeeze it and insert it into the vagina (see Figures 1-4). The correct position of Mirgi is one in which it is not felt. If the system is uncomfortable, the patient should gently adjust its position (e.g., push it slightly further into the vagina) until they feel comfortable. The position of the system in the vagina does not affect its contraceptive effectiveness.
- 6. After 3 weeks, the patient should remove the system from the vagina. This can be done by hooking the finger under the edge of the system or by grasping it with the index and middle fingers and pulling it out (Figure 5). If the patient is unable to remove the system, they should contact their doctor.
- 7. The used system should be disposed of with other household waste, preferably in the original packaging. Mirgi should not be flushed down the toilet.
3.2 Three weeks of use, one week of break
- 1. The system must remain in the vagina for 3 weeks without interruption, counting from the day of insertion.
- 2. After 3 weeks, it should be removed on the same day of the week it was inserted, at approximately the same time. For example, if Mirgi was inserted on a Wednesday at around 10:00 PM, it should be removed on the following Wednesday, 3 weeks later, at around 10:00 PM.
- 3. After removal, the patient should take a 1-week break. During this time, bleeding may occur. It usually starts 2-3 days after removal.
- 4. A new system should be inserted exactly 1 week after the break (on the same day of the week as usual and at approximately the same time), even if bleeding is still present. If the insertion of the new system is delayed by more than 3 hours, its contraceptive effectiveness may be reduced. In such a case, the patient should follow the instructions in section 3.4 "What to do... Procedure when, after a one-week break, the patient has forgotten to insert a new intrauterine therapeutic system".
If Mirgi is used according to the above instructions, subsequent bleedings will occur approximately every month on the same days of the week.
3.3 When to insert the first Mirgi intrauterine therapeutic system
- If the patient did not use a hormonal contraceptive in the previous cycleThe patient should insert Mirgi on the first day of their natural cycle (i.e., the first day of menstruation). Mirgi is effective from the moment of insertion. There is no need to use any additional contraceptive methods. The patient can also start using Mirgi between the 2nd and 5th day of menstruation, but in this case, they should use an additional contraceptive method (such as a male condom) during the first 7 days of using Mirgi.
- If the patient used combined hormonal oral contraceptives in the previous monthThe patient should start using Mirgi no later than the day after the break in taking their current medicine. If their current medicine has tablets that do not contain active substances, the patient should start using Mirgi no later than the day after taking the last tablet that does not contain active substances. If the patient is unsure which tablet this is, they should ask their doctor or pharmacist. The patient should not extend the break in taking their current tablets beyond the recommended period. If the patient has been taking their tablets regularly and is sure they are not pregnant, they can stop taking the tablets on any day and start using Mirgi immediately.
- If the patient used a transdermal system (patch) in the previous monthThe patient should start using Mirgi no later than the day after the break in using the transdermal system. The patient should not extend the break in using the transdermal system beyond the recommended period. If the patient has been using the transdermal system regularly and is sure they are not pregnant, they can stop using the transdermal system on any day and start using Mirgi immediately.
- If the patient used a progestogen-only contraceptive (minipill) in the previous monthThe patient can stop taking the minipill on any day and start using Mirgi the next day at the same time they would have taken the minipill. During the first 7 days of using Mirgi, the patient should also use an additional contraceptive method (such as a male condom).
- If the patient used injections or an implant, or an intrauterine system releasing progestogen [IUS]The patient should start using Mirgi on the day of the next planned injection or on the day the implant or intrauterine system is removed. During the first 7 days of using Mirgi, the patient should also use an additional contraceptive method (such as a male condom).
- After childbirthAfter giving birth, the doctor may recommend using Mirgi only after the first menstrual period. Sometimes, the doctor may recommend starting to use Mirgi earlier; the doctor will advise when. If the patient is breastfeeding and wants to use Mirgi, they should discuss this with their doctor first.
- After a miscarriageAccording to the doctor's instructions.
3.4 What to do…
Procedure in case of accidental expulsion of the system from the vagina
Mirgi may be accidentally expelled from the vagina, e.g., if it was not inserted correctly, during tampon removal, during intercourse, due to constipation, or uterine prolapse. Therefore, the patient should regularly check if the system is in place in the vagina (e.g., before and after intercourse).
3.5 Procedure when the patient wants to stop using the Mirgi drug
The use of the Mirgi drug can be stopped at any time.
If the patient does not want to become pregnant, she should ask her doctor about other contraceptive methods.
If the patient gives up using the Mirgi drug because she wants to become pregnant, she should wait until the first menstruation and only then start trying to become pregnant. This will help determine the date of birth.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
If side effects occur, especially serious and persistent ones or changes in health that the patient considers related to the use of the Mirgi drug, the patient should consult a doctor.
In all women using combined hormonal contraceptives, there is an increased risk of blood clots in the veins (venous thromboembolism) or blood clots in the arteries (arterial thrombosis). For more detailed information on the various risk factors associated with the use of combined hormonal contraceptives, the patient should refer to section 2.
The patient should contact a doctor immediately if any symptoms of angioedema occur, such as: swelling of the face, tongue and/or throat and/or difficulty swallowing or hives that may cause difficulty breathing (see also section "Warnings and precautions").
Women using Mirgi have reported the following side effects:
- Frequent: may occur in up to 1 in 10 women
- abdominal pain, nausea
- vaginal infections by fungi (such as "thrush"); discomfort caused by the presence of the system in the vagina; genital itching; discharge
- headache or migraine; depressive mood; decreased libido
- breast pain; pelvic pain; painful menstruation
- acne
- weight gain
- expulsion of the system
Uncommon: may occur in up to 1 in 100 women
- vision disturbances; dizziness
- bloating; vomiting, diarrhea or constipation
- fatigue, malaise or irritability; mood changes; sudden mood changes
- edema
- urinary tract infections
- problems or pain during urination; urgency to urinate or need to urinate frequently
- discomfort during intercourse, including pain, bleeding, discomfort associated with the presence of the system, felt by the partner
- increased blood pressure
- increased appetite
- back pain; muscle cramps; pain in the lower or upper limbs
- decreased skin sensitivity
- breast tenderness or enlargement; fibrocystic breast disease (lumps that may cause swelling or pain in the breast)
- cervicitis; cervical polyps; cervical ectropion
- changes in menstrual bleeding (e.g. heavy, prolonged, irregular or complete absence of menstruation); pelvic discomfort; premenstrual syndrome; uterine cramps
- vaginal infections (fungal or bacterial); burning sensation, unpleasant odor, pain, discomfort or dryness of the vagina or vulva
- hair loss, rash, itching, hives or hot flashes
- hives
Rare: may occur in up to 1 in 1000 women
- harmful blood clots in a vein or artery, for example:
- in the leg or foot (e.g. deep vein thrombosis)
- in the lungs (e.g. pulmonary embolism)
- heart attack
- stroke
- mini-stroke or transient stroke-like symptoms, known as transient ischemic attack
- blood clots in the liver, stomach, and intestine, kidneys or eye The risk of blood clots may be higher if the patient has other risk factors (see section 2 for more information on risk factors for blood clots and symptoms of blood clots).
- galactorrhea
Unknown (frequency cannot be estimated from the available data)
- chloasma (brownish-yellow spots on the skin, especially on the face)
- partner's genital discomfort (such as irritation, rash, itching)
- inability to remove the therapeutic vaginal system without medical assistance (e.g. due to adhesion to the vaginal wall)
- vaginal wall damage associated with damage to the therapeutic vaginal system
In women using combined hormonal contraceptives, breast cancer and liver tumors have occurred. For more detailed information, see section 2.2 "Important information before using the Mirgi drug", "Tumors".
Very rarely, Mirgi may be damaged. For more information, see section 3.4 "What to do... Procedure in case of insertion of more than one therapeutic vaginal system".
Reporting side effects
If any side effects occur, including any side effects not listed in the leaflet, the patient should tell her doctor or pharmacist. Side effects can be reported directly to the Department of Monitoring of Adverse Reactions to Medicinal Products, Office for Registration of Medicinal Products, Medical Devices and Biocidal Products, Al. Jerozolimskie 181 C, 02-222 Warsaw.
Phone: +48 22 49 21 301, Fax: +48 22 49 21 309, website: https://smz.ezdrowie.gov.pl
Side effects can also be reported to the marketing authorization holder.
Reporting side effects will help to gather more information on the safety of the drug.
5. How to store the Mirgi drug
The drug should be stored out of sight and reach of children.
A doctor should be consulted if a child is exposed to the hormones contained in the Mirgi drug.
This medicinal product does not require special temperature storage conditions.
Store in the original packaging to protect from light.
Do not use the Mirgi drug after the expiry date stated on the box and sachet.
Do not use the Mirgi drug if it has changed color or if there are any signs of deterioration.
The used ring should be disposed of with ordinary household waste, preferably in the original sachet. The Mirgi drug should not be flushed down the toilet. Like other medicines, unused or expired systems should not be disposed of via wastewater or household waste. The patient should ask her pharmacist how to dispose of medicines that are no longer needed. This will help protect the environment.
6. Package contents and other information
What the Mirgi drug contains
- The active substances are etonogestrel and ethinylestradiol. Each ring contains 11.7 mg of etonogestrel and 2.7 mg of ethinylestradiol.
- The other ingredients are ethylene-vinyl acetate copolymer (vinyl acetate 28%), ethylene-vinyl acetate copolymer (vinyl acetate 9%) (a type of plastic that does not dissolve in the body) and magnesium stearate.
Every day for 3 weeks, 0.12 mg of etonogestrel and 0.015 mg of ethinylestradiol are released from the ring.
What the Mirgi drug looks like and what the package contains
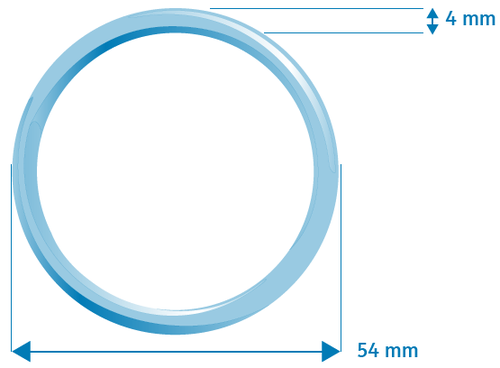
Mirgi is a flexible, transparent, colorless ring with an outer diameter of 54 mm and a cross-sectional diameter of 4 mm. Each ring is packaged in a foil sachet with or without the possibility of multiple closures, depending on the country. The sachet is packaged in a cardboard box. Each box contains 1, 3 or 6 rings.
Not all pack sizes may be available on the market.
Marketing authorization holder and manufacturer
Marketing authorization holder:
Zentiva k.s.
U kabelovny 130
Dolni Mĕcholupy
102 37 Prague 10
Czech Republic
Manufacturer:
Mithra Pharmaceuticals CDMO S.A.
Rue de l’Expansion 57
4400 Flemalle
Belgium
This medicinal product is authorized in the Member States of the European Economic Area under the following names:
Croatia
Etonogestrel/Etinilestradiol Mithra 0.120 mg/0.015 mg for 24 hours, vaginal delivery system
Poland
Mirgi
Austria
MyRing 0.120 mg/0.015 mg for 24 hours, vaginal delivery system
Belgium
Myloop 0.120 mg/0.015 mg for 24 hours, aid for vaginal use
Germany
MYCIRQ 0.120 mg/0.015 mg for 24 hours, vaginal delivery system
Spain
Mithraring 0.120 mg/0.015 mg every 24 hours, vaginal delivery system
France
ETHINYLESTRADIOL/ETONOGESTREL ZENTIVA 15 micrograms /120 micrograms /24 hours, vaginal delivery system
Italy
KIRKOS
Luxembourg
Myloop 0.120 mg/0.015 mg for 24 hours, vaginal delivery system
Netherlands
Etonogestrel/Ethinylestradiol Abbott 0.120 mg/0.015 mg for 24 hours, aid for vaginal use
Date of last revision of the leaflet:July 2023
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- ImporterMithra Pharmaceuticals CDMO S.A.
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to MirgiDosage form: System, (0.12 mg + 0.015 mg)/24 hActive substance: vaginal ring with progestogen and estrogenPrescription requiredDosage form: System, (0.120 mg + 0.015 mg)/24 hActive substance: vaginal ring with progestogen and estrogenManufacturer: N.V. OrganonPrescription requiredDosage form: System, (0.12 mg + 0.015 mg)/24 hActive substance: vaginal ring with progestogen and estrogenPrescription required
Alternatives to Mirgi in other countries
The best alternatives with the same active ingredient and therapeutic effect.
Alternative to Mirgi in Ukraine
Alternative to Mirgi in Spain
Online doctors for Mirgi
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for Mirgi – subject to medical assessment and local rules.






