
Kalium hloratum 15% Kabi
Ask a doctor about a prescription for Kalium hloratum 15% Kabi

How to use Kalium hloratum 15% Kabi
Leaflet accompanying the packaging: information for the user
Potassium Chloride 15% Kabi, 150 mg/ml, concentrate for solution for infusion
Potassium Chloride
Read the leaflet carefully before using the medicine, as it contains important information for the patient.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor, pharmacist, or nurse.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their symptoms are the same as yours.
- If you experience any side effects, including those not listed in this leaflet, please inform your doctor, pharmacist, or nurse. See section 4.
Table of contents of the leaflet
- 1. What is Potassium Chloride 15% Kabi and what is it used for
- 2. Important information before using Potassium Chloride 15% Kabi
- 3. How to use Potassium Chloride 15% Kabi
- 4. Possible side effects
- 5. How to store Potassium Chloride 15% Kabi
- 6. Contents of the packaging and other information
1. What is Potassium Chloride 15% Kabi and what is it used for
This medicine belongs to the group "Intravenous solutions for supplementation: Electrolyte solutions" and is available on prescription.
Potassium Chloride 15% Kabi is indicated for the treatment of potassium deficiency in patients for whom dietary methods or oral treatment are insufficient.
If there is no improvement or the patient feels worse, they should consult a doctor.
2. Important information before using Potassium Chloride 15% Kabi
When not to use Potassium Chloride 15% Kabi
Do not use Potassium Chloride 15% Kabi if you have high levels of potassium in your blood (hyperkalemia).
Warnings and precautions
Before starting treatment with Potassium Chloride 15% Kabi, discuss it with your doctor.
Potassium Chloride 15% Kabi is administered by or under the supervision of a doctor, who will pay attention to the fact that:
- direct injection of concentrated potassium chloride solutions without proper dilution can cause immediate death;
- the medicine should be administered slowly (usually 10 mmol/h, not exceeding 20 mmol/h) and under cardiac monitoring;
- urine output should be monitored in the patient to ensure proper flow;
- electrolyte levels in the blood and acid-base balance should be monitored, and the dose should be adjusted to the individual needs of the patient;
- patients with heart disease, severe fluid deficiency (severe dehydration), muscle cramps caused by dehydration and salt loss due to heat, extensive tissue damage in severe burns, and elderly patients with potential kidney function disorders or other factors predisposing to hyperkalemia should be closely monitored;
- at the start of potassium replacement therapy, glucose infusion solutions should not be administered, as glucose may cause further reduction of potassium levels in the blood;
- if signs of kidney failure occur, intravenous administration of potassium-containing solutions should be discontinued.
The doctor may exercise particular caution and decide whether to administer Potassium Chloride 15% Kabi if you have:
- uncontrolled heart failure during treatment with digitalis glycosides (heart disease medications) and severe or complete atrioventricular block;
- a disease usually associated with increased potassium levels in the blood (hyperkalemia), such as hyperkalemic periodic paralysis (a form of periodic paralysis), sickle cell anemia, adrenal disorders (adrenal insufficiency), kidney function impairment (renal insufficiency), reduced urine output after surgery (postoperative oliguria), shock with hemolysis and/or fluid deficiency (hemolytic shock and/or dehydration), metabolic acidosis (acidic blood), treatment with potassium-sparing diuretics (medications that increase urine output and retain potassium in the blood), increased chloride ion levels in the blood (hyperchloremia).
The doctor should be cautious during intravenous administration, as leakage of the infused fluid outside the vessel (extravasation) can cause tissue necrosis (necrotic tissue damage).
Children and adolescents
The safety and efficacy of potassium chloride in children and adolescents have not been fully established.
Potassium Chloride 15% Kabi and other medicines
Tell your doctor or pharmacist about all the medicines you are taking, have recently taken, or plan to take.
Combinations not recommended (except in cases of severe potassium deficiency):
- potassium-sparing diuretics (medications used to increase urine output that retain potassium in the blood), simple or combined, such as amiloride, spironolactone, triamterene, potassium canrenoate, eplerenone, due to the risk of life-threatening hyperkalemia, especially in patients with kidney function disorders (increased potassium effect);
- angiotensin-converting enzyme (ACE) inhibitors, angiotensin II receptor antagonists, non-steroidal anti-inflammatory drugs (NSAIDs), cyclosporine, tacrolimus, suxamethonium: due to the risk of life-threatening hyperkalemia, especially in patients with kidney function disorders (increased potassium effect);
- blood products, potassium penicillin: due to the possible risk of hyperkalemia caused by the presence of potassium in these medicines.
Combinations possible with special precautions:
- quinidine: potassium may enhance the antiarrhythmic effect of quinidine;
- thiazides, adrenocorticosteroids, glucocorticosteroids, mineralocorticosteroids: the potassium-supplementing effect may be reduced;
- digoxin: hyperkalemia may be dangerous in patients taking digitalis glycosides for heart disease;
- ion exchange resins: serum potassium levels decrease as potassium is replaced by sodium.
In the absence of compatibility studies, this medicine should not be mixed with other medicines.
Incompatibility of Potassium Chloride 15% Kabi, concentrate for solution for infusion, has been reported with the following active substances: amikacin, amphotericin B, dobutamine, fat emulsions, 20% to 25% mannitol solutions, and sodium penicillin G.
Pregnancy, breastfeeding, and fertility
If you are pregnant or breastfeeding, think you may be pregnant, or plan to have a child, ask your doctor or pharmacist for advice before using this medicine.
There are no data or limited data on the use of potassium chloride in pregnant women. The use of Potassium Chloride 15% Kabi may be considered during pregnancy if clinically justified.
Potassium chloride passes into breast milk in such amounts that it may affect newborns/infants being breastfed.
A risk to newborns/infants cannot be excluded.
The attending physician will decide whether to discontinue breastfeeding or discontinue (suspend) the use of Potassium Chloride 15% Kabi, taking into account the benefits of breastfeeding for the child and the benefits of treatment for the woman.
Driving and using machines
There are no data to suggest that this medicine affects the ability to drive and use machines.
3. How to use Potassium Chloride 15% Kabi
Potassium Chloride 15% Kabi will be administered by or under the direct supervision of a doctor, who will carefully control the amount of Potassium Chloride 15% Kabi administered to the patient.
The doctor will decide on the correct dose for the patient.
Recommended dose for adult patients
For intravenous administration only after dilution in an appropriate solution to a maximum potassium chloride concentration of 3 g/l (or 40 mmol/l potassium). In the treatment of severe hypokalemia or diabetic ketoacidosis, higher concentrations may be necessary; in such cases, infusion into a large vein is recommended, and ECG monitoring of the patient is advised.
1 g of potassium chloride corresponds to 13.4 mmol or 524 mg of potassium.
The dose depends on the electrolyte levels in the blood and acid-base balance. Potassium deficiency should be calculated using the following formula:
Potassium deficiency (mmol) = body weight (kg) x 0.2 x 2 x (4.5 mmol/l - serum potassium level)
(The extracellular volume is calculated based on body weight in kg x 0.2)
The standard daily dose is approximately 0.8 to 2 mmol of potassium per kg of body weight.
Usually, the maximum dose used in adult patients should not exceed 150 mmol per day.
Use in children
It is recommended to administer intravenously after dilution in an appropriate solution to a maximum potassium chloride concentration of 3 mmol/kg body weight or 40 mmol/m2 body surface area. In children weighing 25 kg or more, use the same doses as for adult patients.
The maximum dose in children is 3 mmol/kg body weight per day.
In patients with kidney function disorders, the dose should be reduced.
Method of administration
The patient will receive this medicine diluted in a solution for infusion into a vein (intravenous drip). The infusion rate will be slow, and the amount of potassium chloride will depend on the individual needs of the patient.
It is considered that a safe infusion rate is 10 mmol/h. The infusion rate should not exceed 20 mmol/h.
It is recommended to administer using an infusion pump, especially in the case of solutions with higher concentrations.
The doctor will inform you how long the treatment with Potassium Chloride 15% Kabi should last.
If you think that the effect of Potassium Chloride 15% Kabi is too strong or too weak, tell your doctor or pharmacist.
Using a higher dose of Potassium Chloride 15% Kabi than recommended
Overdose due to increased potassium levels in the blood can cause ECG abnormalities, slowed heart rate (bradycardia), irregular heart rhythm with very rapid ventricular fibrillation (ventricular fibrillation), other heart rhythm disorders (arrhythmias) up to cardiac arrest, confusion, fatigue, diarrhea, swallowing disorders, abnormal skin sensations on the hands or feet (paresthesia), breathing difficulties, muscle paralysis, and death.
If any of the above symptoms occur, treatment should be stopped immediately and any food containing potassium and potassium-sparing diuretics (medications used to increase urine output that retain potassium in the blood) should be avoided.
In case of overdose or accidental administration, contact the hospital immediately, stating the name of the medicine and the amount used.
If you experience any of the above symptoms or think you have received too much Potassium Chloride 15% Kabi, inform your doctor or medical staff immediately.
If you have any further questions about the use of this medicine, ask your doctor or pharmacist.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Increased potassium administration can cause increased potassium levels in the blood (hyperkalemia), which can lead to neuromuscular and heart disorders, especially heart rhythm disorders, and even cardiac arrest.
Other side effects:
- Metabolic and nutritional disorders:
- acidic blood (acidosis);
- increased chloride ion levels in the blood (hyperchloremia).
- Vascular disorders:
- blood clots in blood vessels (thrombophlebitis).
- General disorders and administration site conditions:
- nausea;
- pain at the injection site;
- cell death in case of infusion fluid leakage outside the vessel (extravasation);
- vein inflammation in case of local, too high concentration of the solution.
Reporting side effects
If you experience any side effects, including those not listed in this leaflet, please inform your doctor, pharmacist, or nurse. Side effects can be reported directly to the Department of Drug Safety Monitoring of the Office for Registration of Medicinal Products, Medical Devices, and Biocidal Products
Aleje Jerozolimskie 181C
02-222 Warsaw
tel.: +48 22 49 21 301
fax: +48 22 49 21 309
email: [email protected].
Side effects can also be reported to the marketing authorization holder.
Reporting side effects will help to gather more information on the safety of this medicine.
5. How to store Potassium Chloride 15% Kabi
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date stated on the ampoule and carton after "EXP". The expiry date refers to the last day of the month.
There are no special precautions for storage.
Do not use this medicine if the solution is cloudy, contains visible particles, or shows color changes.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. This will help protect the environment.
6. Contents of the packaging and other information
What Potassium Chloride 15% Kabi contains
- The active substance is potassium chloride.
- The other ingredient is water for injections.
1 ml of solution contains 150 mg of potassium chloride (15% w/v), which corresponds to 2 mmol of potassium ions.
5 ml of solution contains 750 mg of potassium chloride (15% w/v), which corresponds to 10 mmol of potassium ions.
10 ml of solution contains 1500 mg of potassium chloride (15% w/v), which corresponds to 20 mmol of potassium ions.
20 ml of solution contains 3000 mg of potassium chloride (15% w/v), which corresponds to 40 mmol of potassium ions.
Ion content:
K
2000 mmol/l
Cl
2000 mmol/l
Theoretical osmolality:
4000 mosm/l
What Potassium Chloride 15% Kabi looks like and contents of the pack
Potassium Chloride 15% Kabi, concentrate for solution for infusion, is a clear, colorless solution.
Potassium Chloride 15% Kabi, concentrate for solution for infusion, is available in the following pack sizes:
20 ampoules of 5 ml
50 ampoules of 5 ml
20 ampoules of 10 ml
50 ampoules of 10 ml
20 ampoules of 20 ml.
Not all pack sizes may be marketed.
Instructions for proper administration
Potassium Chloride 15% Kabi is a sterile solution containing potassium chloride for intravenous infusion. Before use, the medicine should be diluted in at least 50 times the volume of isotonic 0.9% sodium chloride solution (w/v) for intravenous infusion or in another suitable infusion solution.
Before dilution, check the compatibility of potassium chloride with the other infusion solution.
To avoid improper homogenization of the diluted solution, the concentrated solution of Potassium Chloride 15% Kabi should not be added to suspended bottles/bags for infusion.
After adding the concentrated solution to the bottle/bag for infusion, the medicine must be well mixed before use. Therefore, shake the bottle/bag carefully 3 to 5 times to achieve good homogenization. Then, hang the bottle/bag and start the infusion process.
For single use only. Always use after dilution!
After opening the ampoule, its outlet fits exactly to the tip of the Luer or Luer-Lock syringe; therefore, there is no need to use a needle.
Handling instructions
Twist off one ampoule by turning it in the opposite direction to the remaining ones, without touching the tip and neck of the ampoule (1). Shake the ampoule with one movement as shown below to remove the solution from the tip of the ampoule (2). To open the ampoule, twist the tip in the opposite direction to the rest of the ampoule until it breaks off (3). Connect the ampoule to the Luer or Luer-Lock syringe as shown in the picture (4).
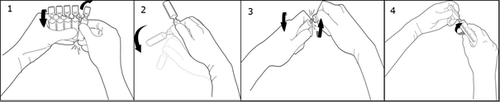
There is no need to use a needle. Always use after dilution!
Marketing authorization holder and manufacturer
Marketing authorization holder
Fresenius Kabi Polska Sp. z o.o.
Aleje Jerozolimskie 134
02-305 Warsaw
Manufacturer
FRESENIUS KABI ESPAÑA, S.A.
C/ Marina 16 – 18, Planta 17
08005 Barcelona
Spain
For more information, contact the marketing authorization holder:
Fresenius Kabi Polska Sp. z o.o.
Aleje Jerozolimskie 134
02-305 Warsaw
tel.: +48 22 345 67 89
This medicinal product is authorized in the Member States of the European Economic Area under the following names:
Belgium
Potassium Chloride Fresenius Kabi 150 mg/ml concentrate for solution for infusion
Estonia
Potassium chloride Kabi 150 mg/ml, concentrate for solution for infusion
Greece
POTASSIUM CHLORIDE/FRESENIUS 150 MG/ML
Spain
Cloruro de potasio Meinsol 2 mEq/ml solution for injection
Ireland
Potassium Chloride 150 mg/ml concentrate for solution for injection or infusion
Lithuania
Potassium chloride Kabi 150 mg/ml concentrate for infusion solution
Latvia
Potassium chloride Kabi 150 mg/ml concentrate for infusion solution preparation
Germany
Kaliumchlorid Kabi 150 mg/ml Konzentrat zur Herstellung einer Infusionslösung
Poland
Kalium chloratum 15% Kabi
Portugal
Cloreto de Potássio Kabi
Romania
Clorură de potasiu Kabi 150 mg/ml
United Kingdom
Potassium Chloride 15% w/v concentrate for solution for infusion
Date of last revision of the leaflet:02.12.2015
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- ImporterFresenius Kabi Espana S.A.
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to Kalium hloratum 15% KabiDosage form: Concentrate, 150 mg/mlActive substance: potassium chloridePrescription not requiredDosage form: Concentrate, -Active substance: electrolytes in combination with other drugsManufacturer: Fresenius Kabi Norge ASPrescription not requiredDosage form: Concentrate, (170.1 mg + 133.5 mg + 14 mg)/mlActive substance: electrolytes in combination with other drugsPrescription not required
Alternatives to Kalium hloratum 15% Kabi in other countries
The best alternatives with the same active ingredient and therapeutic effect.
Alternative to Kalium hloratum 15% Kabi in Ukraine
Alternative to Kalium hloratum 15% Kabi in Spain
Online doctors for Kalium hloratum 15% Kabi
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for Kalium hloratum 15% Kabi – subject to medical assessment and local rules.














