
Flazena
Ask a doctor about a prescription for Flazena

How to use Flazena
Package Leaflet: Information for the Patient
Flazena
(137 micrograms + 50 micrograms)/dose nasal spray, suspension
Azelastine hydrochloride + Fluticasone propionate
Read the package leaflet carefully before using the medicine, as it contains important information for the patient.
- Keep this leaflet, you may need to read it again.
- In case of any doubts, consult a doctor or pharmacist.
- This medicine has been prescribed specifically for you. Do not pass it on to others.
- The medicine may harm another person, even if their symptoms are the same.
- If the patient experiences any side effects, including any not listed in this leaflet, they should tell their doctor or pharmacist. See section 4.
Table of Contents of the Leaflet
- 1. What is Flazena and what is it used for
- 2. Important information before using Flazena
- 3. How to use Flazena
- 4. Possible side effects
- 5. How to store Flazena
- 6. Contents of the pack and other information
1. What is Flazena and what is it used for
Flazena contains two active substances: azelastine hydrochloride and fluticasone propionate.
- Azelastine hydrochloride belongs to a group of medicines called antihistamines. Antihistamines work by preventing the action of substances such as histamine, which the body produces as part of an allergic reaction, and thus reduce the symptoms of allergic rhinitis.
- Fluticasone propionate belongs to a group of medicines called corticosteroids, which reduce inflammation.
Flazena is used to relieve the symptoms of moderate to severe seasonal and perennial allergic rhinitis, if the use of other nasal products containing only an antihistamine or corticosteroid is considered insufficient.
Seasonal and perennial allergic rhinitis is a type of allergic reaction to substances such as plant pollen (hay fever), house dust mites, mold spores, dust, or pet dander.
Flazena relieves allergy symptoms, such as nasal discharge, postnasal drip, sneezing, itching, and nasal congestion.
2. Important information before using Flazena
When not to use Flazena:
- If the patient is allergic to azelastine hydrochloride or fluticasone propionate, or any of the other ingredients of this medicine (listed in section 6).
Warnings and precautions
Before starting to use Flazena, discuss it with your doctor or pharmacist:
- If the patient has recently undergone nasal surgery.
- If the patient has a nasal infection. Nasal infections should be treated with antibacterial or antifungal medicines. Patients who have been treated with medicines for a nasal infection may still be treated for allergies with Flazena.
- If the patient has tuberculosis or an untreated infection.
- If the patient notices a change in vision or has been diagnosed with increased intraocular pressure, glaucoma, and/or cataracts. Patients with these conditions will be closely monitored during treatment with Flazena.
- If the patient has adrenal gland disorders. Caution is advised when switching from systemic steroid therapy to Flazena therapy.
- If the patient has severe liver disease. This increases the risk of systemic side effects.
In these cases, the doctor will decide whether Flazena can be used.
It is important for the patient to take the medicine in the dose prescribed below in section 3 or as advised by the doctor.
Taking nasal corticosteroids in higher doses than recommended may cause adrenal gland suppression, a condition that may lead to weight loss, fatigue, muscle weakness, decreased blood sugar levels, increased salt requirements, joint pain, depression, and darkening of the skin.
In such cases, the doctor may prescribe another medicine during periods of stress or scheduled surgery.
To avoid adrenal gland suppression, the doctor may prescribe the medicine in the smallest dose that will maintain effective control of nasal inflammation symptoms.
If the patient experiences blurred vision or other vision disturbances, they should contact their doctor.
In case of doubt about any of the above situations, the patient should consult their doctor or pharmacist before using Flazena.
Children and adolescents
Flazena is not recommended for use in children under 12 years of age.
In children and adolescents, long-term use of nasal corticosteroids (such as Flazena) may slow growth rate.
The doctor will regularly check the child's growth and ensure that they are taking the medicine in the lowest effective dose.
Flazena and other medicines
The patient should tell their doctor or pharmacist about all medicines they are taking, have recently taken, or might take, including those available without a prescription.
Some medicines may increase the effect of Flazena nasal spray, and the doctor may recommend close monitoring if the patient is taking such medicines (including certain HIV medicines: ritonavir, cobicistat, and antifungal medicines: ketoconazole).
If the patient is taking sedatives or central nervous system depressants, they should not use Flazena.
Pregnancy and breastfeeding
If the patient is pregnant or breastfeeding, thinks they may be pregnant, or plans to have a baby, they should consult their doctor or pharmacist before using this medicine.
Driving and using machines
Flazena has a minor influence on the ability to drive and use machines.
Rarely, fatigue and dizziness may occur, which may be caused by the disease itself or the use of Flazena.
In such cases, the patient should not drive or operate machinery.
The patient should be aware that drinking alcohol may increase this effect.
Flazena contains benzalkonium chloride
This medicine contains 14 micrograms of benzalkonium chloride per dose.
Benzalkonium chloride may cause irritation or swelling inside the nose, especially if used for a long time.
3. How to use Flazena
Flazena should always be used as directed by the doctor.
In case of doubt, the patient should consult their doctor or pharmacist.
To get the most benefit from the treatment, Flazena must be used regularly.
The patient should avoid contact with the eyes.
Adults and adolescents (12 years and older)
- One dose should be administered into each nostril in the morning and evening.
Use in children under 12 years
- Flazena is not recommended for use in children under 12 years of age.
Use in patients with renal and hepatic impairment
- There are no data on the use of Flazena in patients with renal and hepatic impairment.
Method of administration
Nasal administration.
The patient should carefully read the instructions below and use the medicine only as directed.
INSTRUCTIONS FOR USE
Preparing the nasal spray
- 1. Gently shake the bottle for 5 seconds, moving it up and down, then remove the protective cap.
- 2. If the nasal spray is used for the first time, prime the pump by releasing a dose into the air.
- 3. Prime the pump by placing two fingers on either side of the pump and the thumb on the bottom of the bottle.
- 4. Press and release the pump 6 times until a fine mist is produced (see figure a).
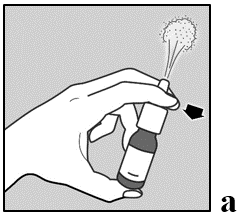
- 5. The pump is now primed and ready for use.
- 6. If the nasal spray has not been used for more than 7 days, re-prime the pump by pressing and releasing it once.
Using the nasal spray
- 1. Gently shake the bottle for 5 seconds, moving it up and down, then remove the protective cap.
- 2. Blow the nose to clear the nostrils.
- 3. Tilt the head forward towards the toes. Do not tilt the head back.
- 4. Hold the bottle upright and gently place the tip of the dosing nozzle into the nostril.
- 5. Close the other nostril with a finger, press the pump quickly once, and at the same time, take a gentle breath in (see figure b).
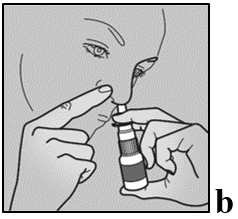
- 6. Breathe out through the mouth.
- 7. Repeat the same steps for the other nostril.
- 8. After administration, take gentle breaths and do not tilt the head back. This will prevent the medicine from entering the throat and causing an unpleasant taste.
- 9. After each use, wipe the tip of the dosing nozzle with a clean tissue or cloth and replace the protective cap.
- 10. In case of blockage, do not pierce the tip of the dosing nozzle. Wash the tip with water.
It is essential to use the dose prescribed by the doctor.
The patient should only use the dosing recommended by the doctor.
Duration of treatment
Flazena can be used for a long time.
The duration of treatment should correspond to the period during which allergy symptoms occur.
Using a higher dose of Flazena than recommended
In case of using too much nasal spray, there is a small risk of complications.
The patient should consult their doctor if they are concerned or have been taking a higher dose than recommended for a long time.
If someone, especially a child, accidentally swallows Flazena, they should immediately contact a doctor or the nearest hospital emergency department.
Missing a dose of Flazena
The patient should use the nasal spray as soon as they remember and then use the next dose at the usual time.
The patient should not use a double dose to make up for a missed dose.
Stopping treatment with Flazena
The patient should not stop using Flazena without consulting their doctor, due to the risk of reduced treatment effectiveness.
In case of any further doubts about using this medicine, the patient should consult their doctor or pharmacist.
4. Possible side effects
Like all medicines, Flazena can cause side effects, although not everybody gets them.
Very common side effects (may affect more than 1 in 10 people):
- Nosebleeds
Common side effects (may affect up to 1 in 10 people):
- Headache
- Bitter taste in the mouth, especially if the patient tilts their head back during administration of the nasal spray. This taste should disappear if the patient drinks a non-alcoholic beverage a few minutes after using the medicine
- Unpleasant odor
Uncommon side effects (may affect up to 1 in 100 people):
- Mild irritation inside the nose. This may cause a mild stinging sensation, itching, or sneezing
- Dryness in the nose, cough, dry throat, or throat irritation
Rare side effects (may affect up to 1 in 1000 people):
- Dry mouth
Very rare side effects (may affect up to 1 in 10,000 people):
- Dizziness or drowsiness
- Cataracts, glaucoma, or increased eye pressure, which may cause vision loss and/or eye redness and pain. These side effects have been reported after long-term use of nasal sprays containing fluticasone propionate.
- Skin and nasal mucosa damage
- General malaise, feeling tired, exhausted, or weak
- Rash, itching, or redness of the skin, itchy blisters on the skin
- Bronchospasm (narrowing of the airways)
The patient should immediately seek medical attention if they experience any of the following symptoms:
- Swelling of the face, lips, tongue, or throat, which may cause difficulty swallowing and/or breathing, and sudden appearance of a skin rash. These may be symptoms of a severe allergic reaction. Note: these symptoms are very rare.
Side effects with unknown frequency (frequency cannot be estimated from the available data):
- Blurred vision
- Ulcers of the nasal mucosa
In case of using the medicine in high doses for a long time, systemic side effects (affecting the whole body) may occur.
The likelihood of these side effects is much lower when using nasal corticosteroids than when taking oral corticosteroids.
These side effects may vary between patients and after using different corticosteroid medicines (see section 2).
Nasal corticosteroids may affect the normal production of hormones in the body, especially when taken for a long time in high doses.
In children and adolescents, these side effects may cause growth retardation.
In rare cases, a decrease in bone density (osteoporosis) has been observed when corticosteroids were used nasally for a long time.
Reporting side effects
If the patient experiences any side effects, including any not listed in the leaflet, they should tell their doctor or pharmacist.
Side effects can be reported directly to the Department of Drug Safety, Office for Registration of Medicinal Products, Medical Devices, and Biocidal Products:
Al. Jerozolimskie 181 C, 02-222 Warsaw, tel.: +48 22 49 21 301, fax: +48 22 49 21 309,
website: https://smz.ezdrowie.gov.pl
Side effects can also be reported to the marketing authorization holder.
By reporting side effects, more information can be gathered on the safety of the medicine.
5. How to store Flazena
The medicine should be stored out of sight and reach of children.
Do not use this medicine after the expiry date stated on the label and carton:
EXP. The expiry date refers to the last day of the month.
Do not store in the refrigerator or freeze.
Shelf life after first opening the bottle: the unused medicine should be discarded 6 months after opening the nasal spray.
Medicines should not be disposed of via wastewater or household waste.
The patient should ask their pharmacist how to dispose of medicines that are no longer needed.
This will help protect the environment.
6. Contents of the pack and other information
What Flazena contains
- The active substances are azelastine hydrochloride and fluticasone propionate.
- Each gram of suspension contains 1000 micrograms of azelastine hydrochloride and 365 micrograms of fluticasone propionate.
After each actuation of the pump (0.14 g), 137 micrograms of azelastine hydrochloride (= 125 micrograms of azelastine) and 50 micrograms of fluticasone propionate are delivered.
- The other ingredients are disodium edetate, glycerol, microcrystalline cellulose, sodium croscarmellose, polysorbate 80, benzalkonium chloride, phenylethyl alcohol, and purified water.
What Flazena looks like and contents of the pack
Flazena is a white to almost white, homogeneous suspension.
Flazena is available in a brown glass bottle with a pump spray, nasal applicator, and protective cap, in a cardboard box.
The 25 mL bottle contains 23 g of nasal spray suspension (at least 120 doses).
Flazena is available in:
- Packaging containing 1 bottle with 23 g of nasal spray suspension.
Marketing authorization holder:
Zentiva, k.s.
U kabelovny 130
Dolní Měcholupy
102 37 Prague 10
Czech Republic
Manufacturer:
FARMEA
10, rue Bouché Thomas
ZAC d’Orgemont
49007 Angers
France
Farmaclair
440 Avenue Du Général De Gaulle
14200 Hérouville-Saint-Clair
France
For further information, please contact the marketing authorization holder:
Zentiva Polska Sp. z o.o.
ul. Bonifraterska 17
00-203 Warsaw
Tel.: +48 22 375 92 00
This medicine is authorized in the Member States of the European Economic Area under the following names:
Denmark, Czech Republic, Norway, Slovakia, Sweden: Lutonaze
Germany: Azelastin/Fluticason Zentiva 137 Mikrogramm/50 Mikrogramm pro Sprühstoß Nasenspray, Suspension
France: AZELASTINE CHLORHYDRATE / FLUTICASONE PROPIONATE ZENTIVA 137 microgrammes/50 microgrammes, suspension pour pulvérisation nasale
Lithuania: AZAVIN 137 mikrogramai/50 mikrogramų/dozėje nosies purškalas (suspensija)
Poland: Flazena
Romania: Lutonaze 137 micrograme/50 micrograme/doza spray nazal, suspensie
Hungary: Lutonaze 137 mikrogramm/50 mikrogramm szuszpenziós orrspray
Italy: Azelastina e Fluticasone Zentiva
Date of last revision of the leaflet:January 2025
- Country of registration
- Active substance
- Prescription requiredYes
- ImporterFarmaclair FARMEA
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to FlazenaDosage form: Aerosol, (137 mcg + 50 mcg)/doseActive substance: fluticasone, combinationsPrescription requiredDosage form: Aerosol, (137 mcg + 50 mcg)/ doseActive substance: fluticasone, combinationsPrescription requiredDosage form: Aerosol, (137 mcg + 50 mcg)/nasal doseActive substance: fluticasone, combinationsPrescription required
Alternatives to Flazena in other countries
The best alternatives with the same active ingredient and therapeutic effect.
Alternative to Flazena in Spain
Alternative to Flazena in Ukraine
Online doctors for Flazena
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for Flazena – subject to medical assessment and local rules.








