

Azecort

Ask a doctor about a prescription for Azecort

How to use Azecort
Leaflet accompanying the packaging: patient information
Azecort
(137 micrograms + 50 micrograms)/dose, nasal spray, suspension
Azelastine hydrochloride + Fluticasone propionate
Read the leaflet carefully before using the medicine, as it contains important information for the patient.
- Keep this leaflet, you may need to read it again.
- In case of any doubts, consult a doctor or pharmacist.
- This medicine has been prescribed specifically for this person. Do not pass it on to others. The medicine may harm another person, even if their symptoms are the same.
- If the patient experiences any side effects, including any not listed in this leaflet, they should tell their doctor or pharmacist. See section 4.
Table of contents of the leaflet
- 1. What is Azecort and what is it used for
- 2. Important information before using Azecort
- 3. How to use Azecort
- 4. Possible side effects
- 5. How to store Azecort
- 6. Contents of the packaging and other information
1. What is Azecort and what is it used for
Azecort contains two active substances: azelastine hydrochloride and fluticasone propionate.
- Azelastine hydrochloride belongs to a group of medicines called antihistamines. Antihistamines work by preventing the action of substances such as histamine, which the body produces as part of an allergic reaction, and thus reduce the symptoms of allergic rhinitis.
- Fluticasone propionate belongs to a group of medicines called corticosteroids, which reduce inflammation.
Azecort is used for the symptomatic treatment of severe seasonal allergic rhinitis in adults.
2. Important information before using Azecort
When not to use Azecort:
- If the patient is allergic to azelastine hydrochloride or fluticasone propionate, or any of the other ingredients of this medicine (listed in section 6).
Warnings and precautions
Before starting treatment with Azecort, the patient should discuss the following with their doctor or pharmacist:
- if the patient has recently undergone nasal surgery
- if the patient has a nasal infection. Nasal infections should be treated with antibacterial or antifungal medicines. Patients who have been treated with medicines for a nasal infection may continue to treat seasonal allergies with Azecort.
- if the patient has tuberculosis or an untreated infection
- if the patient is taking or has recently taken other medicines containing corticosteroids
- if the patient notices a change in vision or has a history of increased intraocular pressure, glaucoma, and/or cataracts. Patients with these conditions will be closely monitored during treatment with Azecort.
- if the patient has adrenal gland problems. Caution is advised when switching from systemic steroid treatment to Azecort therapy.
- if the patient has severe liver disease. This may increase the risk of systemic side effects.
In these cases, the doctor will decide whether Azecort can be used.
Children and adolescents
Azecort is not recommended for use in children and adolescents under 18 years of age.
Azecort and other medicines
The patient should tell their doctor or pharmacist about all medicines they are currently taking or have recently taken, as well as any medicines they plan to take.
Pregnancy and breastfeeding
If the patient is pregnant or breastfeeding, thinks they may be pregnant, or plans to have a baby, they should consult their doctor or pharmacist before using this medicine.
Driving and using machines
Azecort has a minor influence on the ability to drive and use machines.
Azecort contains benzalkonium chloride
This medicine contains 14 micrograms of benzalkonium chloride per actuation, which corresponds to 0.014 mg/0.14 g. Benzalkonium chloride may cause irritation or swelling inside the nose, especially if used for a long time.
3. How to use Azecort
This medicine should always be used as directed by the doctor or pharmacist.
Adults
- The recommended dose is one actuation into each nostril in the morning and evening.
Use in children and adolescents under 18 years of age
- This medicine is not recommended for use in children and adolescents under 18 years of age.
Use in patients with renal and hepatic impairment
- There are no data on the use of Azecort in patients with renal and hepatic impairment.
Method of administration
Nasal use (intranasal use).
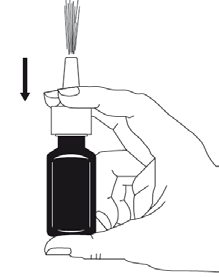


Duration of treatment
Azecort may be used for a long time. The duration of treatment should correspond to the period during which allergy symptoms occur.
Overdose
In case of overdose, there is a low risk of complications. The patient should consult their doctor if they are concerned or if they have taken a higher dose than recommended for a long time.
Missed dose
The patient should use the nasal spray as soon as they remember, and then use the next dose at the usual time. They should not use a double dose to make up for a missed dose.
Stopping treatment
The patient should not stop using Azecort without consulting their doctor, as this may lead to a lack of treatment efficacy.
4. Possible side effects
Like all medicines, Azecort can cause side effects, although not everybody gets them.
Very common side effects (may affect more than 1 in 10 people):
- Nosebleeds
Common side effects (may affect up to 1 in 10 people):
- Headache
- Bitter taste in the mouth, especially if the patient tilts their head back during nasal spray use. This taste should disappear if the patient drinks a non-alcoholic beverage a few minutes after using the medicine.
- Unpleasant odor
Uncommon side effects (may affect up to 1 in 100 people):
- Mild irritation inside the nose. This may cause a mild stinging sensation, itching, or sneezing.
- Dryness in the nose, cough, dry throat, or throat irritation.
Rare side effects (may affect up to 1 in 1,000 people):
- Dry mouth
Very rare side effects (may affect up to 1 in 10,000 people):
- Dizziness or drowsiness
- Cataracts, glaucoma, or increased eye pressure, which may cause vision loss and/or eye redness and pain. These side effects have been reported after long-term use of nasal sprays containing fluticasone propionate.
- Skin and nasal mucosa damage
- General malaise, fatigue, weakness, or exhaustion.
- Rash, itching, or redness of the skin, itchy blisters on the skin.
- Bronchospasm (narrowing of the airways).
In case of any of the following symptoms, the patient should seek medical attention immediately:
- Swelling of the face, lips, tongue, or throat, which may cause difficulty swallowing or breathing, and sudden appearance of skin rash.These may be symptoms of a severe allergic reaction. Note: These symptoms are very rare.
Side effects with unknown frequency (cannot be estimated from the available data):
- Blurred vision
- Ulcers of the nasal mucosa
5. How to store Azecort
The medicine should be stored out of sight and reach of children.
6. Contents of the packaging and other information
What Azecort contains
The active substances are azelastine hydrochloride and fluticasone propionate.
What Azecort looks like and contents of the pack
Azecort is a white, homogeneous suspension.
Azecort is available in a glass bottle with a pump spray, nasal applicator, and cap in a cardboard box.
- Packaging with 1 bottle containing 6.4 g of nasal spray suspension.
- Packaging with 1 bottle containing 23 g of nasal spray suspension.
Marketing authorisation holder
Viatris Healthcare Limited
Manufacturer
MEDA Pharma GmbH & Co. KG
Mylan Hungary Kft
H-2900 Komárom
Viatris Healthcare Sp. z o.o.
This medicine is authorised in the Member States of the European Economic Area and in the United Kingdom (Northern Ireland) under the following names:
| Austria | Synaze 137 Mikrogramm/50 Mikrogramm pro Sprühstoß Nasenspray, Suspension | Latvia | Bileni 137 mikrogrami/50 mikrogrami devā deguna aerosols, suspensija |
| Bulgaria | Azecort | Liechtenstein | Synaze 137 Mikrogramm/50 Mikrogramm pro Sprühstoß Nasenspray, Suspension |
| Cyprus | Bileni Ρινικό εκνέφωμα | Lithuania | Bileni 137 mikrogramai/50 mikrogramu/ dozëje nosies purškalas (suspensija) |
| Czech Republic | Dymistalin 137 mikrogramů / 50 mikrogramů, nosní sprej, suspenze | Luxembourg | Dyvistanil 137 mcg/50 mcg/dose Solution pour pulvérisation nasale |
| Denmark | Azecort, næsespray, suspension | Malta | Azecort Nasal Spray |
| Estonia | Bileni | Norway | Synaze |
| France | Synaze, Suspension pour pulvérisation nasale | Portugal | Azecort 137 microgramas / 50 microgramas por aplicação Suspensão para pulverização nasal |
| Germany | Dyvistanil Nasenspray 137 Mikrogramm/50 Mikrogramm pro Sprühstoß Nasenspray, Suspension | Romania | Synaze 137 micrograme / 50 micrograme /doza spray nazal suspensie |
| Greece | Bileni Ρινικό εκνέφωμα | Slovakia | Azecort nosová aerodisperzia |
| Hungary | Bileni szuszpenziós orrspray | Slovenia | Synaze 137 mikrogramov / 50 mikrogramov na vpih pršilo za nos, suspenzija |
| Iceland | Azecort 137 míkróg / 50 míkróg/skammt nefúđi, dreifa | Spain | Synaze 137 microgramos/50 microgramos/applicacion suspensión pulverización nasal |
| Ireland | Azecort 137 micrograms / 50 micrograms per actuation, Nasal Spray Suspension | Sweden | Dyvistalin 125 mikrogram + 50 mikrogram/sprayning nässpray, suspension |
| Italy | Dygaro 137 Microgrammi/50 Microgrammi/Erogazione Spray Nasale, Sospensione | United Kingdom (Northern Ireland) | Azelastine / Fluticasone 137 micrograms / 50 micrograms per actuation Nasal Spray, Suspension |
Date of last revision of the leaflet: 08/2024
- Country of registration
- Active substance
- Prescription requiredYes
- ImporterHaupt Pharma Amareg GmbH MADAUS GmbH Meda Pharma GmbH & Co. KG Mylan Hungary Kft.
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to AzecortDosage form: Aerosol, (137 mcg + 50 mcg)/doseActive substance: fluticasone, combinationsPrescription requiredDosage form: Aerosol, (137 mcg + 50 mcg)/ doseActive substance: fluticasone, combinationsPrescription requiredDosage form: Aerosol, (137 mcg + 50 mcg)/nasal doseActive substance: fluticasone, combinationsPrescription not required
Alternatives to Azecort in other countries
The best alternatives with the same active ingredient and therapeutic effect.
Alternative to Azecort in Spain
Alternative to Azecort in Ukraine
Online doctors for Azecort
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for Azecort – subject to medical assessment and local rules.








