
Duosol zavieraiomci 4 mmol/l potasu
Ask a doctor about a prescription for Duosol zavieraiomci 4 mmol/l potasu

How to use Duosol zavieraiomci 4 mmol/l potasu
Leaflet attached to the packaging: patient information
Duosol containing 4 mmol/l of potassium, solution for hemofiltration
You should carefully read the contents of the leaflet before using the medicine, as it contains
important information for the patient
- You should keep this leaflet, so that you can read it again if you need to.
- If you have any doubts, you should consult a doctor or pharmacist.
- If the patient experiences any side effects, including any side effects not listed in this leaflet, they should inform their doctor or pharmacist. See section 4.
Table of contents of the leaflet
- 1. What is Duosol containing 4 mmol/l of potassium and what is it used for
- 2. Important information before using Duosol containing 4 mmol/l of potassium
- 3. How to use Duosol containing 4 mmol/l of potassium
- 4. Possible side effects
- 5. How to store Duosol containing 4 mmol/l of potassium
- 6. Contents of the packaging and other information
1. What is Duosol containing 4 mmol/l of potassium and what is it used for
Duosol containing 4 mmol/l of potassium is a solution for hemofiltration. This medicine is intended for use in patients with acute renal failure, whose kidneys are not able to remove waste products from the blood. The process of continuous hemofiltration involves removing waste products from the body that are normally excreted by the kidneys. The solution balances fluid and provides replacement of lost salts (electrolytes).
2. Important information before using Duosol containing 4 mmol/l of potassium
Duosol containing 4 mmol/l of potassium should not be used if:
- the patient has abnormally high potassium levels in the blood (hyperkalemia);
- the patient has abnormally low acid levels in the blood (metabolic alkalosis).
Hemofiltration treatment should not be used in the following cases:
- renal failure combined with very intense metabolic processes (hyperkatabolism); in such cases, the accumulated waste products cannot be removed for a longer period through hemofiltration;
- insufficient blood flow from the venous access;
- any conditions in which there is an increased risk of bleeding, as the patient is receiving medications that prevent blood clotting (systemic anticoagulation).
Warnings and precautions
Before starting treatment with Duosol containing 4 mmol/l of potassium, you should discuss it with your doctor or pharmacist.
Before and during hemofiltration, you should monitor blood pressure, fluid balance, electrolyte balance, acid-base balance, and kidney function. You should regularly check blood sugar and phosphate levels.
Before and during hemofiltration, you should also monitor potassium levels in the blood.
Duosol containing 4 mmol/l of potassium and other medicines
You should tell your doctor or pharmacist about all medicines you are currently taking or have recently taken, as well as any medicines you plan to take.
The levels of other medicines in the blood may decrease during hemofiltration, and your doctor will take this into account.
By using the appropriate doses of the hemofiltration solution and careful monitoring, you can avoid interactions with other medicines.
The following interactions will be considered:
- Infusions given as part of intensive medical care may change the composition of the patient's blood and fluid status.
- The toxic effects of some medications used to treat heart weakness (medicines containing digitalis) may not be visible when potassium or magnesium levels are too high or calcium levels are too low. If these levels are corrected by hemofiltration, toxic effects may occur and cause, for example, heart rhythm disturbances. If the patient has low potassium levels or high calcium levels in the blood, digitalis may have toxic effects at lower doses than usually used in treatment.
- Administration of vitamin D and calcium-containing medications may increase the risk of high calcium levels in the blood (hypercalcemia).
- Additional use of sodium bicarbonate may increase the risk of abnormally low acid levels in the blood (metabolic alkalosis).
Pregnancy, breastfeeding, and fertility
If you are pregnant or breastfeeding, think you may be pregnant, or plan to have a child, you should consult your doctor or pharmacist before receiving this medicine.
There are no data on the use of hemofiltration solutions in pregnant women. However, since all the components of this medicine are naturally occurring substances that only replace the same substances lost from the body during hemofiltration, no risks are expected for the child during pregnancy and breastfeeding, and no effect on fertility is expected.
Driving and using machines
This medicine is usually given to patients who are immobilized in a hospital/dialysis unit. This excludes driving and using machines.
3. How to use Duosol containing 4 mmol/l of potassium
This medicine will be given to you only under the supervision of a doctor with experience in hemofiltration techniques.
Your doctor will decide on the appropriate dose for you, taking into account your clinical condition, body weight, and metabolic conditions. Unless otherwise recommended, for patients of all age groups, a filtration rate of 20-25 ml/kg body weight per hour is recommended to ensure the elimination of substances that are normally excreted in the urine.
The patient is given a ready-to-use hemofiltration solution through the tubes of the hemofiltration device (so-called extracorporeal circuit) using an infusion pump.
Treatment of acute renal failure is carried out for a limited period and ends when kidney function is restored.
Using a higher dose of Duosol containing 4 mmol/l of potassium than recommended
No life-threatening cases have been reported after administration of the recommended doses of this medicine. If necessary, administration can be stopped at any time.
Incorrect fluid balance may lead to the presence of too much or too little water in the body (overhydration or dehydration). These conditions are characterized by changes in blood pressure or heart rate.
Overdose of bicarbonate may occur when too much hemofiltration solution is administered. This may lead to abnormally low acid levels in the blood (metabolic alkalosis), lower levels of calcium dissolved in the blood (decrease in ionized calcium levels), or muscle cramps (tetany).
Overdose may cause congestive heart failure and (or) pulmonary congestion and may cause changes in electrolyte balance and acid-base balance.
Your doctor will decide on the appropriate treatment.
If you have any further doubts about the use of this medicine, you should consult your doctor or pharmacist.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
No side effects related to the use of this medicine have been reported so far, but the following side effects are possible. The frequency of these side effects is unknown (cannot be estimated from the available data):
Excess or deficiency of water in the body (overhydration or dehydration), abnormal electrolyte levels, low phosphate levels in the blood (hypophosphatemia), high blood sugar levels (hyperglycemia), abnormally low acid levels in the blood (metabolic alkalosis), too high or too low blood pressure (hypertension, hypotension), nausea, vomiting, and muscle cramps.
Reporting side effects
After the product has been placed on the market, it is important to report any suspected side effects. This allows for continuous monitoring of the benefit-risk balance of the product. Healthcare professionals should report any suspected side effects via the Department of Adverse Reaction Monitoring of Medicinal Products, Medical Devices, and Biocidal Products, Al. Jerozolimskie 181C, 02-222 Warsaw
Phone: +48 22 49 21 301
Fax: +48 22 49 21 309
website: https://smz.ezdrowie.gov.pl
Side effects can also be reported to the marketing authorization holder.
Reporting side effects will allow for more information to be collected on the safety of this medicine.
5. How to store Duosol containing 4 mmol/l of potassium
The medicine should be stored out of sight and reach of children.
Do not use this medicine after the expiry date stated on the bag and carton after:
“Expiry date”. The expiry date refers to the last day of the given month.
Storage conditions
Do not store above 25°C. Do not store in the refrigerator or freeze.
Storage conditions after preparation of the ready-to-use solution
The mixed product should be used immediately. The product after mixing retains physical and chemical stability for 24 hours at 25°C.
6. Contents of the packaging and other information
What Duosol containing 4 mmol/l of potassium contains
| Active substances: | Smaller chamber Electrolyte solution | Larger chamber Bicarbonate solution | ||
| 555 ml contains | per 1000 ml | 4445 ml contains | per 1000 ml | |
| sodium chloride | 2.34 g | 4.21 g | 27.47 g | 6.18 g |
| potassium chloride | 1.49 g | 2.68 g | ||
| calcium chloride dihydrate | 1.10 g | 1.98 g | ||
| magnesium chloride hexahydrate | 0.51 g | 0.91 g | ||
| glucose monohydrate equivalent to glucose anhydrous | 5.49 g 5.0 g | 9.90 g 9.0 g | ||
| sodium bicarbonate | 15.96 g | 3.59 g | ||
| Electrolytes: | [mmol/ chamber] | [mmol/l] | [mmol/ chamber] | [mmol/l] |
| Na+ | 40.0 | 72 | 660 | 149 |
| K+ | 20.0 | 36.0 | ||
| Ca2+ | 7.5 | 13.5 | ||
| Mg2+ | 2.5 | 4.5 | ||
| Cl- | 95.0 | 171 | 470 | 106 |
| HCO3- | 190 |
| ||
| theoretical osmolality [mOsm/l] | 347 | 297 | ||
Composition of the ready-to-use hemofiltration solution after mixing:
1000 ml of the ready-to-use hemofiltration solution contains [mmol/l]:
Sodium
140
Potassium 4.0
Calcium
1.5
Magnesium
0.5
Chloride
113
Bicarbonate
35.0
glucose anhydrous
5.6 (equivalent to 1.0 g)
Theoretical osmolality [mOsm/l]
300
pH
7.0-8.0
Other ingredients are:
Electrolyte solution (smaller chamber)
hydrochloric acid 25% (for pH adjustment), water for injections
Bicarbonate solution (larger chamber)
carbon dioxide (for pH adjustment), water for injections
What Duosol containing 4 mmol/l of potassium looks like and what the pack contains
Hemofiltration solution
Clear and colorless solution, free from visible particles
This medicine is supplied in a dual-chamber bag. By mixing the two solutions after opening the partition between the chambers, a ready-to-use hemofiltration solution is obtained.
2 bags of 5000 ml (dual-chamber bags, 4445 ml and 555 ml) in a cardboard carton.
Marketing authorization holder and manufacturer
- B. Braun Avitum AG Schwarzenberger Weg 73-79 34212 Melsungen Germany
This medicinal product is authorized in the Member States of the European Economic Area under the following names:
Czech Republic:
Duosol with 4 mmol/l potassium
Greece:
Duosol with 4 mmol/l Potassium Διάλυμα αιμοδιήθησης
Estonia:
Duosol with 4 mmol/l potassium, hemofiltration solution
Finland:
Duosol with 4 mmol/l potassium hemofiltration solution
Italy:
Duosol with 4 mmol/l of potassium solution for hemofiltration
Latvia:
Duosol with 4 mmol/l potassium solution for hemofiltration
Lithuania:
Duosol K 4 hemofiltration solution
Germany, Luxembourg:
Duosol with 4 mmol/l potassium hemofiltration solution
Poland:
Duosol containing 4 mmol/l of potassium
Slovenia:
Duosol with 4 mmol/l potassium solution for hemofiltration
Spain:
Priosol with 4 mmol/l of potassium solution for hemofiltration
Netherlands:
Duosol with 4 mmol/l potassium, solution for hemofiltration
United Kingdom:
Duosol with 4 mmol/l potassium solution for hemofiltration
Date of last revision of the leaflet: 05.05.2023
---------------------------------------------------------------------------------------------------------------------------------
Information intended for healthcare professionals only:
Instructions for preparing the ready-to-use hemofiltration solution
Before use, the container and solution should be visually inspected. The hemofiltration solution should only be used if the container (outer protective packaging and dual-chamber bag), the partition between the chambers, and the connections are undamaged and intact, and the solution is clear, colorless, and free from visible particles.
The outer protective packaging should be removed immediately before use.
- 1. Remove the outer protective packaging.
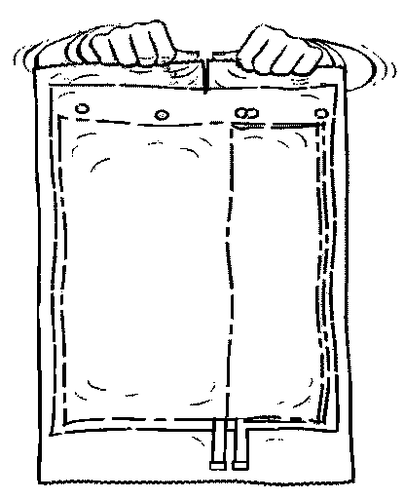
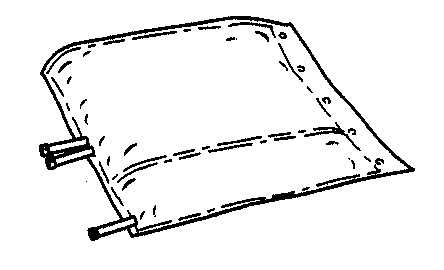
- 2. Unfold the bag and place it on a clean, flat surface.
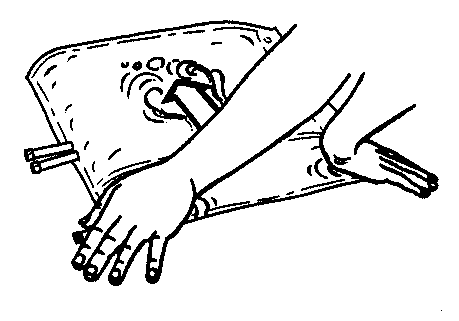
- 3. Press the smaller chamber of the bag with both hands to fully open the partition between the chambers along its entire length.
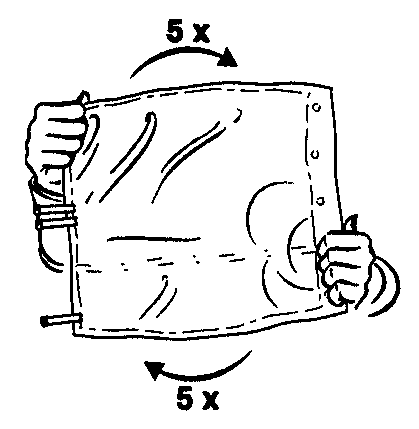
- 4. Ensure thorough mixing of the contents by rotating the bag five times in both directions.
Administration of the ready-to-use hemofiltration solution
The hemofiltration solution should be warmed to approximately body temperature using an integrated or external warmer. Under no circumstances should the solution be administered if it is below room temperature.
During the use of this medicinal product, rare cases of white calcium carbonate precipitate have been observed in the tubes, especially near the pump and heating device. Therefore, during hemofiltration, the solution in the infusion tubes should be visually monitored every 30 minutes to ensure that the solution in the tubes is clear and free from precipitates.
Precipitates may also occur with significant delay after the start of treatment. If precipitates are observed, the solution and infusion tubes should be replaced immediately, and the patient should be closely monitored.
For single use only. Unused solution and any damaged containers should be discarded.
- Country of registration
- Prescription requiredNo
- Manufacturer
- ImporterB. Braun Avitum AG
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to Duosol zavieraiomci 4 mmol/l potasuDosage form: Solution, 9 mg/mlActive substance: sodium chloridePrescription requiredDosage form: Solution, 100 mg/mlActive substance: dextranManufacturer: Fresenius Kabi Italia S.r.L.Prescription not requiredDosage form: Concentrate, -Active substance: electrolytes in combination with other drugsManufacturer: Fresenius Kabi Norge ASPrescription not required
Alternatives to Duosol zavieraiomci 4 mmol/l potasu in other countries
The best alternatives with the same active ingredient and therapeutic effect.
Alternative to Duosol zavieraiomci 4 mmol/l potasu in Ukraine
Alternative to Duosol zavieraiomci 4 mmol/l potasu in Spain
Online doctors for Duosol zavieraiomci 4 mmol/l potasu
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for Duosol zavieraiomci 4 mmol/l potasu – subject to medical assessment and local rules.














