
Diphereline Sr 3,75

Ask a doctor about a prescription for Diphereline Sr 3,75

How to use Diphereline Sr 3,75
Leaflet accompanying the packaging: information for the user
Diphereline SR 3.75
3.75 mg
Powder and solvent for prolonged-release suspension for injection
Triptorelinum
Read the leaflet carefully before using the medicine, as it contains important information for the patient.
- Keep this leaflet, you may need to read it again.
- In case of any doubts, consult a doctor or pharmacist.
- This medicine has been prescribed specifically for you. Do not pass it on to others. The medicine may harm another person, even if their symptoms are the same.
- If the patient experiences any side effects, including any side effects not listed in this leaflet, they should tell their doctor or pharmacist. See section 4.
Table of contents of the leaflet
- 1. What is Diphereline SR 3.75 and what is it used for
- 2. Important information before using Diphereline SR 3.75
- 3. How to use Diphereline SR 3.75
- 4. Possible side effects
- 5. How to store Diphereline SR 3.75
- 6. Contents of the packaging and other information
1. What is Diphereline SR 3.75 and what is it used for
This medicine contains triptorelin. Triptorelin belongs to a group of medicines known as gonadotropin-releasing hormone (GnRH) analogues. One of its actions is to reduce the production of sex hormones in the body.
Prolonged administration of triptorelin, after initial stimulation, causes inhibition of gonadotropin secretion (FSH and LH), the ultimate effect of which is inhibition of testicular and ovarian function.
Another mechanism of action of the medicine is also indicated: a direct effect on the testes and ovaries by reducing the sensitivity of their receptors to the hormone that increases the release of sex hormones (gonadoliberyn).
Diphereline SR 3.75 is indicated in:
- Prostate cancer Treatment of prostate cancer when testosterone levels need to be reduced to castration levels. The beneficial effect of treatment is more pronounced and more frequent if the patient has not received any other hormonal treatment before.
- Breast cancer in women before and after menopause, when hormonal treatment is indicated
- Central precocious puberty (before the age of 8 in girls and before the age of 10 in boys).
- Endometriosis of the genital and external organs (stages I to IV) Treatment should not be continued for more than 6 months (see section 4.8). A second treatment cycle with triptorelin or another GnRH analogue is not recommended.
- Uterine fibroids Treatment of uterine fibroids for surgical preparation or in patients who are not eligible for surgery.
- Infertility in women
Adjuvant treatment in combination with gonadotropin administration (hMG, FSH, hCG) to induce ovulation, in preparation for in vitro fertilization and embryo transfer to the uterus.
2. Important information before using Diphereline SR 3.75
When not to use Diphereline SR 3.75
- if the patient is allergic to triptorelin, gonadorelin (GnRH), other GnRH analogues or any of the other ingredients of this medicine (listed in section 6)
- if premature gonadotropin-independent sexual development has occurred
- if the patient is pregnant or breastfeeding
Warnings and precautions
Before starting treatment with Diphereline SR 3.75, discuss it with your doctor or pharmacist.
In adults, long-term treatment with triptorelin may cause bone weakness (osteoporosis) associated with an increased risk of bone fractures. You should inform your doctor if you have any of the following risk factors, as your doctor may recommend taking bisphosphonates (medicines used to treat osteoporosis) to treat bone mass loss.
Risk factors may include:
- osteoporosis in the patient or their immediate family;
- alcohol abuse, smoking, malnutrition;
- long-term use of medicines that may cause bone mass loss, such as medicines used to treat epilepsy or corticosteroids (such as hydrocortisone, prednisolone).
There have been reports of depression, including severe depression, in patients taking Diphereline SR 3.75. If depressive moods occur while taking Diphereline SR 3.75, you should inform your doctor.
If seizures occur, you should immediately inform your doctor. Seizures have been reported in patients taking triptorelin or similar medicines. They occurred in patients with previously diagnosed epilepsy and without it.
During treatment with Diphereline SR 3.75, a pituitary tumor (benign tumor) may be revealed, which the patient was not aware of. Symptoms include sudden headache, vomiting, vision disturbances, and eye muscle paralysis.
Men
In the case of anticoagulant therapy, bruising may occur at the injection site.
At the beginning of treatment, an increase in testosterone levels in the body may be observed. This may cause an exacerbation of symptoms related to the tumor. In such a case, you should contact your doctor. Your doctor may prescribe an additional medicine (antiandrogen) to prevent the exacerbation of these symptoms.
If the patient has urinary tract obstruction or spinal cord compression due to tumor spread, the doctor will closely monitor the patient during the first few weeks of treatment. In case of difficulty urinating, bone pain, lower limb weakness, or numbness, you should immediately contact your doctor, who will assess the patient's condition and initiate appropriate treatment.
Tryptorelin does not induce further reduction of testosterone levels in patients after orchiectomy (testicle removal).
The results of diagnostic tests of gonadal pituitary and reproductive organ function performed during or after treatment with Diphereline SR 3.75 may be misleading.
You should inform your doctor if you have heart or blood vessel diseases, including arrhythmias, or if you are taking medicines used to treat arrhythmias. The risk of arrhythmias may be increased during treatment with Diphereline SR 3.75.
Medicines that lower testosterone levels may cause changes in the ECG related to arrhythmias (prolongation of the QT interval).
You should inform your doctor if you have diabetes and/or heart disease.
Women
Uterine fibroids and endometriosis
In the first month of treatment, vaginal bleeding may occur. Then, under normal conditions, menstruation will stop. If bleeding occurs after the first month of treatment, you should tell your doctor.
Menstruation should return 2-3 months after the last injection. When treatment is not used due to infertility, during the first month of treatment and after the last injection, a non-hormonal contraceptive method should be used.
If the patient has submucous myomas (benign tumors of the uterine muscle located under the mucous membrane), within the first 6-10 weeks after starting treatment, Diphereline SR 3.75 may cause bleeding when myomas degenerate. In case of heavy or unusual bleeding or pain, you should immediately contact your doctor.
Infertility in women
The treatment effect may be significantly increased in a small number of predisposed patients.
The treatment effect may vary between different patients after using the same doses, and in some cases between different cycles in the same patient.
The use of gonadotropins in combination with this medicine for the treatment of infertility may cause ovarian enlargement or hyperstimulation of the ovaries, manifested by pelvic and/or abdominal pain and difficulty breathing. In such a case, you should immediately contact your doctor.
Breast cancer in women before and after menopause
The use of Diphereline SR 3.75 may be useful in cases where other treatment methods have not led to a clinical response or have lost their effectiveness.
Children
In girls with central precocious puberty, vaginal bleeding may occur in the first month of treatment.
You should inform your doctor if you have a progressing brain tumor. This may affect your doctor's decision on the treatment method.
After stopping treatment with Diphereline SR 3.75 mg in a patient, signs of puberty will appear.
In girls, menstruation usually occurred within a year after stopping treatment.
The doctor should rule out the possibility that precocious puberty is caused by other diseases.
The amount of minerals in the bones decreases during treatment, but returns to normal values after its discontinuation.
After stopping treatment, a hip joint disease (juvenile epiphyseal separation) may occur, causing stiffness, laxity, and/or severe pain in the groin radiating to the thigh. In such a case, you should consult a doctor.
If a child experiences severe or recurrent headaches, vision problems, and ringing or buzzing in the ears, you should immediately contact a doctor (see section 4).
In case of concerns related to the above situations, you should discuss them with your doctor.
Diphereline SR 3.75 and other medicines
Tell your doctor or pharmacist about all medicines you are currently taking or have recently taken, as well as any medicines you plan to take.
Diphereline SR 3.75 may affect the action of other medicines used to treat arrhythmias (e.g., quinidine, procainamide, amiodarone, sotalol) or may increase the risk of arrhythmias when taken with other medicines (e.g., methadone used to treat pain and as part of detoxification in the case of drug addiction), moxifloxacin (an antibiotic), antipsychotic medicines used to treat severe mental illnesses).
Pregnancy and breastfeeding
If you are pregnant or breastfeeding, think you may be pregnant, or plan to have a child, consult your doctor before using this medicine.
Diphereline SR 3.75 should not be used during pregnancy and breastfeeding.
You should not take Diphereline SR 3.75 while trying to conceive (unless Diphereline SR 3.75 is part of infertility treatment).
Driving and using machines
During treatment, dizziness, fatigue, or vision problems may occur, e.g., blurred vision. These symptoms may be side effects of treatment or result from the underlying disease. In case of any of these side effects, you should not drive vehicles or operate any machines.
Diphereline SR 3.75 contains sodium
The medicine contains less than 1 mmol (23 mg) of sodium per dose, i.e., the medicine is considered "sodium-free".
3. How to use Diphereline SR 3.75
The medicine will be administered by your doctor or nurse in a dose suitable for you, depending on the indication for which it was prescribed.
Treatment of children: the dose should be adjusted according to body weight.
Children with a body weight over 30 kg: one intramuscular injection every 4 weeks (28 days).
Children with a body weight between 20 and 30 kg: two-thirds of the dose at intramuscular injection every 4 weeks (28 days), i.e., administer two-thirds of the volume of the prepared suspension.
Children with a body weight below 20 kg: half of the dose at intramuscular injection every 4 weeks (28 days), i.e., administer half of the volume of the prepared suspension.
You should not stop treatment without consulting your doctor.
Using a higher dose of Diphereline SR 3.75 than recommended
In case of overdose, symptomatic treatment is recommended.
4. Possible side effects
Like all medicines, Diphereline SR 3.75 can cause side effects, although not everybody gets them.
In rare cases, a severe allergic reaction (angioedema, anaphylactic reaction) may occur. If symptoms such as difficulty swallowing or breathing, dizziness, swelling of the lips, face, throat, or tongue, or rash occur, you should immediately contact your doctor.
Men
Many of the side effects are expected to be caused by changes in testosterone levels in the body. These effects include hot flashes, impotence, and decreased libido.
Very common- side effects that occur in more than 1 in 10 patients
- Hot flashes
- Weakness
- Intensive sweating
- Back pain
- Pins and needles in the lower limbs
- Decreased sexual desire
- Impotence
Common- side effects that occur in no more than 1 in 10 patients
- Nausea, dry mouth
- Pain, bruising, redness, and swelling at the injection site
- Muscle and bone pain, pain in the arms and legs, swelling (fluid accumulation in tissues), abdominal pain
- High blood pressure
- Allergic reaction
- Weight gain
- Dizziness, headache
- Loss of sexual desire, depression, mood changes
Uncommon- side effects that occur in no more than 1 in 100 patients
- Increased platelet count
- Palpitations
- Ringing in the ears, dizziness, blurred vision
- Abdominal pain, constipation, diarrhea, vomiting
- Sluggishness, chills, drowsiness, pain
- Changes in some test results (including increased liver enzyme values), elevated blood pressure
- Weight loss
- Loss of appetite, increased appetite, gout (severe pain and swelling of joints, most commonly affecting the big toe), diabetes, high blood lipid levels
- Joint pain, muscle cramps, muscle weakness, muscle pain, swelling of the ankles, feet, or toes, bone pain
- Numbness and tingling
- Sleep disturbances, irritability
- Breast enlargement in men, breast pain (breast tenderness), decreased testicle size, testicle pain
- Shortness of breath
- Acne, hair loss, itching, rash, redness of the skin, hives
- Frequent urination at night, urination disorders
- Nosebleeds
Rare- side effects that occur in no more than 1 in 1000 patients
- Red or purple skin discoloration
- Unusual sensations in the eye, blurred or disturbed vision
- Feeling of fullness in the abdomen, bloating, taste disturbances
- Chest pain
- Difficulty standing
- Flu-like symptoms, fever
- Severe allergic reaction (anaphylactic reaction) that can cause dizziness or breathing difficulties, swelling of the face, throat, or tongue
- Rhinitis, pharyngitis
- Joint stiffness, joint swelling, musculoskeletal stiffness, degenerative joint inflammation
- Memory loss
- Confusion, decreased activity, excitement
- Shortness of breath when lying down
- Blisters on the skin
- Low blood pressure
Side effects reported after the medicine was placed on the market (frequency not known):
- General malaise, fever
- Seizures
- High blood pressure
- Severe allergic reaction causing swelling of the face, tongue, and throat, dizziness, or breathing difficulties (angioedema, anaphylactic shock)
- Changes in some blood test results (including increased enzyme activity)
- Muscle weakness
- Confusion, unusual sensations in the eye, and/or vision disturbances
- Amenorrhea
- Diarrhea
- In the case of a pituitary tumor, increased risk of bleeding into the pituitary
- Hives
During infertility treatment, gonadotropins in combination with Diphereline SR 3.75 may cause pelvic and/or abdominal pain and shortness of breath. In such a case, you should immediately tell your doctor.
Symptoms for which endometriosis treatment was justified (pelvic pain, painful menstruation) may worsen at the beginning of treatment but should improve within one to two weeks. This may happen even if the treatment is effective. You should immediately inform your doctor.
Children
Very common- side effects that occur in more than 1 in 10 patients
- In girls, vaginal bleeding may occur in the first month of treatment
Common- side effects that occur in no more than 1 in 10 patients
- Abdominal pain
- Pain, bruising, redness, and swelling at the injection site
- Headache, hot flashes
- Weight gain, acne
- Allergic reaction
Uncommon- side effects that occur in no more than 1 in 100 patients
- Blurred vision
- Vomiting, constipation, nausea
- Obesity
- Neck pain, chest pain
- Mood changes, malaise
- Nosebleeds
- Itching, rash, or hives
Side effects reported after the medicine was placed on the market (frequency not known):
- High blood pressure
- Seizures
- Abnormal vision
- Severe allergic reaction causing swelling of the face, tongue, and throat, dizziness, or breathing difficulties (angioedema, anaphylactic shock)
- Changes in some blood test results, including hormone levels
- Muscle pain
- Mood disturbances, depression, nervousness
- Idiopathic intracranial hypertension (increased pressure inside the skull around the brain, characterized by headache, double vision, and other vision disturbances, and ringing or buzzing in the ears)
Reporting side effects
If you experience any side effects, including any side effects not listed in this leaflet, you should tell your doctor or pharmacist, or nurse. Side effects can be reported directly to the Department of Adverse Reaction Monitoring of Medicinal Products, Medical Devices, and Biocidal Products
Al. Jerozolimskie 181C
02-222 Warsaw
Phone: +48 22 49 21 301
Fax: +48 22 49 21 309
Website: https://smz.ezdrowie.gov.pl
Side effects can also be reported to the marketing authorization holder.
By reporting side effects, you can help provide more information on the safety of this medicine.
5. How to store Diphereline SR 3.75
Store the medicine out of sight and reach of children.
Do not use this medicine after the expiry date stated on the carton. The expiry date refers to the last day of the month.
Expiry date (EXP)
Batch number (Lot)
Store below 25°C.
6. Contents of the packaging and other information
What Diphereline SR 3.75 contains
The active substance of the medicine is triptorelin in the form of triptorelin acetate. One vial contains 3.75 mg of triptorelin.
The other ingredients are:
- The vial with powder contains lactide-coglycolide polymer, mannitol, sodium carmellose, polysorbate 80
- The ampoule with solvent contains mannitol, water for injections
What Diphereline SR 3.75 looks like and what the pack contains
Powder and solvent for prolonged-release suspension for injection.
Vial with powder and ampoule with solvent.
Box containing 1 vial and 1 ampoule with 1 syringe (made of polypropylene) and 2 needles.
Marketing authorization holder and manufacturer
Marketing authorization holder
Ipsen Pharma
65 Quai Georges Gorse
92100 Boulogne Billancourt
France
Manufacturer
Ipsen Pharma Biotech
Parc d’Activités du Plateau de Signes
Chemin départemental nr 402
83870 Signes
France
To obtain more detailed information about this medicine, please contact the local representative of the marketing authorization holder:
Ipsen Poland Sp. z o.o.
ul. Chmielna 73
00-801 Warsaw
phone: 22 653 68 00
fax: 22 653 68 22
Date of last revision of the leaflet:October 2024
<-------------------------------------------------------------------------------------------------------------------------
Information intended only for healthcare professionals:
Dosage and administration
Prostate cancer
Two dosing regimens are possible:
- subcutaneous injection of Diphereline 0.1 mg with immediate release once daily for 7 consecutive days, and then one intramuscular injection of Diphereline SR 3.75 on the 8th day, every 4 weeks.
- or intramuscular injection of Diphereline SR 3.75 every 4 weeks.
In patients with prostate cancer with metastases resistant to castration, not undergoing surgical treatment, receiving triptorelin and eligible for treatment with androgen biosynthesis inhibitors, triptorelin treatment should be continued.
Breast cancer in women before and after menopause, when hormonal treatment is indicated
One injection of Diphereline SR 3.75 every 4 weeks. Do not stop treatment without consulting your doctor.
Central precocious puberty (before the age of 8 in girls and before the age of 10 in boys)
Treatment of children with triptorelin should be carried out under the comprehensive supervision of a pediatric endocrinologist or a pediatrician or endocrinologist with experience in the treatment of central precocious puberty.
- Children with a body weight over 30 kg: one intramuscular injection every 4 weeks (28 days).
- Children with a body weight between 20 and 30 kg: two-thirds of the dose at intramuscular injection every 4 weeks (28 days), i.e., administer two-thirds of the volume of the prepared suspension.
- Children with a body weight below 20 kg: half of the dose at intramuscular injection every 4 weeks (28 days), i.e., administer half of the volume of the prepared suspension.
Endometriosis
Treatment should be started within the first 5 days of the menstrual cycle.
- Dosing regimen: one injection of Diphereline SR 3.75 every 4 weeks.
- Duration of treatment: depends on the initial stage of endometriosis and the clinical changes observed during treatment (functional and anatomical). Generally, treatment should be carried out for at least 4 months and no more than 6 months. A second treatment cycle with triptorelin or any other GnRH analogue is not recommended.
Uterine fibroids
Treatment must be started within the first 5 days of the menstrual cycle. Diphereline SR 3.75 should be administered every 4 weeks. In patients scheduled for surgery, treatment is carried out for 3 months. In patients not eligible for surgery, treatment is limited to 6 months.
Infertility in women
One vial of Diphereline SR 3.75 is administered intramuscularly on the 2nd day of the cycle. Gonadotropin administration should be started after decreased pituitary sensitivity has been achieved (estradiol levels in serum below 50 pg/ml), usually about 15 days after Diphereline SR 3.75 injection.
In the case of women, the specialist must carefully assess the justification for prolonged administration of the preparation for more than 6 months to determine whether the expected benefit outweighs the potential side effects associated with prolonged inhibition of estrogen production, particularly in bones.
You should strictly follow your doctor's instructions and not stop treatment without consulting your doctor.
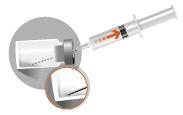
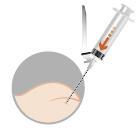
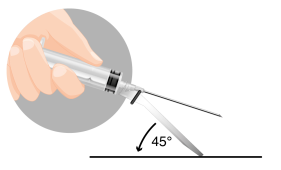
The medicine is administered by intramuscular injection after prior preparation.
Note: It is essential to perform the injection strictly according to the instructions for use.
INSTRUCTIONS FOR RECONSTITUTION OF THE MEDICINE
| |
| Preparation of the patient involves disinfecting the injection site on the buttock. The patient should be prepared for injection before reconstitution of the medicine, as the medicine should be administered immediately after its reconstitution. | |
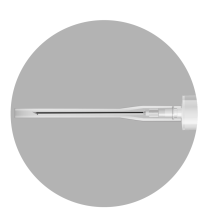 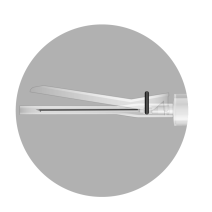 | |
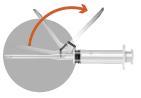
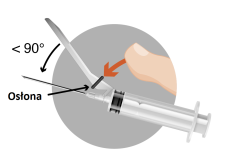
The packaging contains 2 needles:
| |
2a
|  |
2b
|  |
2c
| |
2d
|  |
|  |
|  |
|  |
| Sposób A Sposób B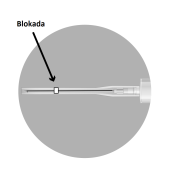 |
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- ImporterIpsen Pharma Biotech SAS
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to Diphereline Sr 3,75Dosage form: Solution, 0.1 mg/mlActive substance: triptorelinManufacturer: Ferring GmbHPrescription requiredDosage form: Powder, 3.75 mgActive substance: triptorelinManufacturer: Ferring GmbHPrescription requiredDosage form: Powder, 0.1 mgActive substance: triptorelinManufacturer: Ipsen Pharma Biotech SASPrescription required
Alternatives to Diphereline Sr 3,75 in other countries
The best alternatives with the same active ingredient and therapeutic effect.
Alternative to Diphereline Sr 3,75 in Ukraine
Alternative to Diphereline Sr 3,75 in Spain
Online doctors for Diphereline Sr 3,75
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for Diphereline Sr 3,75 – subject to medical assessment and local rules.











