
SUPREFACT 1 mg/ml INJECTABLE SOLUTION

How to use SUPREFACT 1 mg/ml INJECTABLE SOLUTION
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the User
Suprefact 1 mg/ml Solution for Injection
Buserelin
Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
- Keep this leaflet, you may need to read it again. If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the Package Leaflet
- What is Suprefact and what is it used for
- What you need to know before you use Suprefact
- How to use Suprefact
- Possible side effects
- Storage of Suprefact
- Contents of the pack and other information
1. What is Suprefact and what is it used for
It belongs to a group of medicines called hormones, as the active ingredient buserelin is a substance capable of simulating the action of a hormone. The result is the inhibition of the release of gonadotropins (sex hormones) in men and the stimulation of ovulation in women.
In men, this medicine is prescribed to treat advanced prostate cancer when it is capable of responding to hormonal treatment, but it is not applicable after total orchiectomy (removal of both testicles by surgery).
In women, this medicine is prescribed to stimulate the ovulation process, as complementary treatment in an in vitro fertilization program.
2. What you need to know before you use Suprefact
Do not use Suprefact
- if you are allergic to buserelin or any of the other components of this medicine (listed in section 6).
- if you have a tumor disease that does not respond to hormonal treatment.
- if you have undergone orchiectomy (removal of testicles by surgery).
- if you are pregnant or breastfeeding (see section "Pregnancy and breastfeeding").
Warnings and Precautions
Consult your doctor or pharmacist before starting to use Suprefact:
- since it is recommended to use an anti-androgen medicine (a medicine that neutralizes the action of testosterone) together with Suprefact, starting its administration a few days before.
- if you have known metastases (e.g. in the spinal cord), as the combined use of an anti-androgen medicine may prevent complications derived from treatment with Suprefact (see section 4).
- if you have risk factors for cardiovascular disease (heart disease) or diabetes.
- if you have any heart or blood vessel condition or are being treated for it, including medicines to control heart rhythm (arrhythmias). The risk of heart rhythm problems may increase when using Suprefact Injection.
- if you have high blood pressure, as blood pressure control may be affected by treatment (see section 4).
- if you have a decreased number of red blood cells or feel an increase in fatigue (anemia).
- if you are diabetic, as metabolic control may be affected by treatment (see section 4).
- if you have a bone metabolic disease or other risk factors for osteoporosis such as chronic alcohol abuse, smoking, family history of osteoporosis or are on long-term treatment with anticonvulsants or corticosteroids.
- if you have ever suffered from depression, as there is a risk of relapse or worsening of an existing depression (see section 4).
- if you are administered Suprefact to stimulate ovulation. Before starting administration, a pregnancy test is recommended.
- if you are receiving Suprefact solution for injection together with gonadotropins to stimulate ovulation, as a series of symptoms called "ovarian hyperstimulation syndrome" may appear, which requires strict supervision by your doctor.
Depression has been reported in patients using Suprefact, which could be severe. If you are using Suprefact and develop a depressive mood, inform your doctor.
Other Medicines and Suprefact
Tell your doctor or pharmacist if you are using, have recently used, or might use any other medicines.
In particular, consult your doctor:
If you take Suprefact with antidiabetics, their action may decrease.
If you take Suprefact with sex hormones, your doctor will establish the appropriate dose to achieve an optimal overall effect.
Suprefact may interfere with some medicines used to treat heart rhythm problems (e.g. quinidine, procainamide, amiodarone, and sotalol) or may increase the risk of heart rhythm problems when used with other medicines (e.g. methadone (used for pain relief and detoxification from other medicines), moxifloxacin (an antibiotic), antipsychotics (used to treat severe mental illnesses).
Pregnancy, Breastfeeding, and Fertility
Suprefact should not be administered to pregnant women (see "Do not use Suprefact").
Suprefact should not be used in breastfeeding women (see "Do not use Suprefact").
Consult your doctor or pharmacist before using this medicine.
Children and Adolescents
Its use is not recommended in children because its effect on this population is not known.
Patients Over 65 Years Old
No specific studies have been conducted in this population.
Driving and Using Machines
You should keep in mind that concentration and reaction time may be altered by the appearance of dizziness, and this may affect your ability to drive vehicles or operate machinery.
Consult your doctor or pharmacist before using any medicine.
Suprefact contains benzyl alcohol and sodium
This medicine contains 10 mg of benzyl alcohol in each ml of injectable solution.
Benzyl alcohol may cause allergic reactions.
Consult your doctor or pharmacist if you are pregnant or breastfeeding. This is because large amounts of benzyl alcohol can accumulate in your body and cause adverse effects (metabolic acidosis).
Consult your doctor or pharmacist if you have liver or kidney disease. This is because large amounts of benzyl alcohol can accumulate in your body and cause adverse effects (metabolic acidosis).
This medicine contains less than 23 mg of sodium (1 mmol) per milliliter of injectable solution; that is, it is essentially "sodium-free".
3. How to Use Suprefact
Follow exactly the administration instructions of this medicine indicated by your doctor. In case of doubt, consult your doctor or pharmacist again.
Remember to use your medicine.
It is recommended to distribute the injections throughout the day, at the most regular time intervals possible.
In prostate cancer, you will be administered 0.5 ml of Suprefact injectable solution 3 times a day by subcutaneous route. From the eighth day of treatment with Suprefact injectable solution, your doctor will indicate that you continue with Suprefact nasal.
If your doctor has prescribed Suprefact injectable solution as complementary treatment to stimulate ovulation, you will be administered 0.3 ml of solution 2 times a day by subcutaneous route. The treatment will start on the first or second day of the cycle, or around day 21 if pregnancy has been ruled out.
If you think the action of Suprefact is too strong or too weak, tell your doctor or pharmacist.
Your doctor will indicate the duration of your treatment with Suprefact. Do not stop your treatment before, as it could worsen your disease.
If you use more Suprefact than you should
If you have used more Suprefact than you should, consult your doctor, pharmacist, or call the Toxicology Information Service, phone 91 562 04 20, indicating the medicine and the amount used. It is recommended to take the package and the package leaflet of the medicine to the healthcare professional.
In case of overdose, you may feel tiredness, headache, nervousness, hot flashes, dizziness, nausea, abdominal pain, swelling in the legs, and breast pain.
If you forget to use Suprefact
Do not use a double dose to make up for forgotten doses.
If you have any other doubts about the use of this medicine, ask your doctor or pharmacist.
4. Possible Side Effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Hot flashes, impotence, breast enlargement in men (without pain), and swelling in the ankles and feet may appear.
You may have discomfort or local reactions at the injection site.
In women, menstrual-like bleeding may appear in the first weeks of treatment. Occasionally, it may appear in the following weeks of treatment. It can also cause hot flashes, increased sweating, vaginal dryness, pain during intercourse, decreased sexual desire, and, in long-term treatments, decreased bone mass.
In women, the following symptoms have sometimes occurred, although they have not been related to the use of Suprefact: breast enlargement or reduction with discomfort, brittle nails, acne, dry skin, occasional vaginal discharge, occasional edema (fluid retention) in the face and extremities.
In women, milk secretion from the breasts, stomach pain, abdominal pain, paresthesia (tingling) in the arms and legs, dry eyes (which could cause irritation if contact lenses are used) may also appear.
In the initial phase of treatment, ovarian cysts may appear, although this would not have a negative effect on stimulation for ovulation induction.
Tell your doctor or pharmacist if any of the following side effects is serious or lasts more than a few days:
Common (may affect up to 1 in 10 people)
- Mood changes and depression (in long-term treatment)
- Headache
- Pain or local reactions at the injection site.
Uncommon (may affect up to 1 in 100 people)
- Allergic reactions such as skin redness, itching, or rash
- Mood changes and depression (in short-term treatment)
- Drowsiness, dizziness
- Constipation
- Tiredness.
Rare (may affect up to 1 in 1,000 people)
- Allergic asthma with dyspnea (difficulty breathing) and in isolated cases may cause a severe allergic reaction that can put your life at risk
- Nervousness, emotional instability, anxiety
- Sleep disturbances, memory and concentration disturbances
- Palpitations
- Increased blood pressure if you are hypertensive
- Nausea, vomiting, diarrhea
- Increased or decreased hair and body hair.
Very Rare (may affect up to 1 in 10,000 people)
- Appearance of a benign tumor in the pituitary gland (mucosa that lines the inside of the nose). It is also possible that the tumor pain increases slightly
- Decrease in the number of platelets (blood cells involved in clotting) or white blood cells in the blood
- Increased thirst and changes in appetite. Additionally, you may observe a decrease in glucose tolerance, if you are diabetic, you should consult your doctor (see "Warnings and Precautions")
- Visual disturbances, such as blurred vision and feeling of pressure behind the eyes
- Tinnitus and other hearing disorders
- Muscle discomfort and pain. You may also be at a higher risk of bone fractures due to decreased bone density.
Frequency Not Known (cannot be estimated from the available data)
- Changes in the electrocardiogram ECG (QT interval prolongation)
- In women with uterine fibroids, degeneration of them
- When used to treat prostate cancer, LHRH analogs may increase the risk of cardiovascular disease (such as heart attack and stroke), diabetes, or anemia (decrease in the number of red blood cells that causes you to feel tired).
Reporting of Side Effects
If you experience any side effect, consult your doctor or pharmacist, even if it is a possible side effect not listed in this leaflet. You can also report them directly through the Spanish Medicines Monitoring System: https://www.notificaram.es. By reporting side effects, you can contribute to providing more information on the safety of this medicine.
5. Storage of Suprefact
Store below 25°C. Do not freeze. Store in the outer packaging to protect from light.
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date which is stated on the carton after EXP. The expiry date is the last day of the month stated.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. These measures will help protect the environment.
6. Contents of the Pack and Other Information
Composition of Suprefact
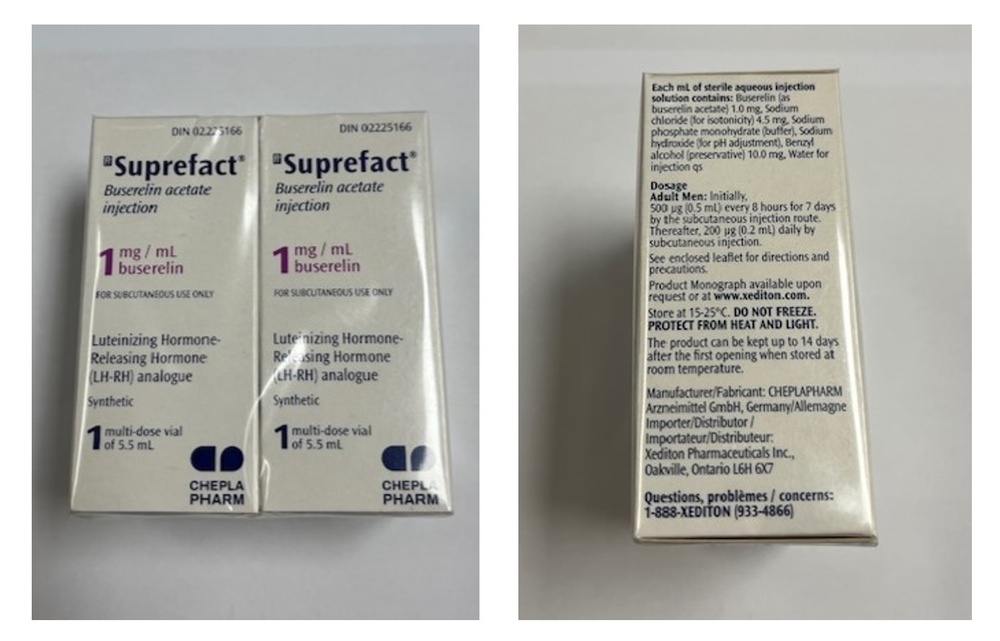
- The active substance is buserelin (as buserelin acetate). Each vial contains 5.5 ml of injectable solution, at a concentration of 1.05 mg/ml of buserelin acetate (equivalent to 1.0 mg/ml of buserelin).
- The other components are: benzyl alcohol, sodium dihydrogen phosphate dihydrate, sodium chloride, sodium hydroxide, and water for injectable preparations.
Appearance of the Product and Contents of the Pack
Suprefact is a clear injectable solution. It is presented in a transparent glass vial with a gray rubber stopper and an aluminum/propylene cap.
Each carton contains 2 vials.
Marketing Authorization Holder and Manufacturer
Marketing Authorization Holder:
CHEPLAPHARM Arzneimittel GmbH
Ziegelhof 24
17489 Greifswald
Germany
Manufacturer:
Sanofi-Aventis Deutschland GmbH
Brüningstraße 50
Frankfurt am Main
Germany
Or
Famar Health Care Services Madrid, S.A.U.
Avda. de Leganés, 62
28923 Alcorcón (Madrid)
Spain
Local Representative
Laboratorios Rubió, S.A.
Industria, 29
Pol. Ind. Comte de Sert
08755 Castellbisbal (Barcelona)
Spain
Date of Last Revision of this Leaflet: February 2016
Other Sources of Information
Detailed information on this medicine is available on the website of the Spanish Agency for Medicines and Health Products (AEMPS) http://www.aemps.gob.es/.
- Country of registration
- Average pharmacy price53.45 EUR
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to SUPREFACT 1 mg/ml INJECTABLE SOLUTIONDosage form: IMPLANT, 9.45 mgActive substance: buserelinManufacturer: Cheplapharm Arzneimittel GmbhPrescription requiredDosage form: INJECTABLE, 42 mgActive substance: leuprorelinManufacturer: Accord Healthcare S.L.U.Prescription requiredDosage form: INJECTABLE, 0.1 mgActive substance: triptorelinManufacturer: Ipsen Pharma S.A.Prescription required
Online doctors for SUPREFACT 1 mg/ml INJECTABLE SOLUTION
Discuss questions about SUPREFACT 1 mg/ml INJECTABLE SOLUTION, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions












