
Decapeptil Depot

Ask a doctor about a prescription for Decapeptil Depot

How to use Decapeptil Depot
Package Leaflet: Information for the Patient
DECAPEPTYL DEPOT,
3.75 mg, powder and solvent for suspension for injection
Triptorelin
Read the package leaflet carefully before using the medicine, as it contains important information for the patient.
Keep this package leaflet, you may need to read it again.
In case of further questions, consult a doctor.
This medicine has been prescribed to you by a doctor and should not be given to others. It may harm them, even if their symptoms are the same as yours.
If you experience any side effects, including those not listed in this package leaflet, inform your doctor. See section 4.
Contents of the package leaflet:
- 1. What is Decapeptyl Depot and what is it used for
- 2. Important information before using Decapeptyl Depot
- 3. How to use Decapeptyl Depot
- 4. Possible side effects
- 5. How to store Decapeptyl Depot
- 6. Contents of the pack and other information
1. What is Decapeptyl Depot and what is it used for
Decapeptyl Depot contains triptorelin (as triptorelin acetate). One of its actions is to reduce the production of sex hormones by the body.
Decapeptyl Depot is used:
In men:
for the treatment of advanced prostate cancer,
for the diagnosis of hormone-dependent prostate cancer (assessment of indications for hormone treatment).
In women:
to reduce ovarian hormone levels for:
reducing the size of uterine fibroids (commonly known as myomas), which are benign tumors that develop from the smooth muscle layer of the uterus,
treating endometriosis (the growth of uterine tissue outside the uterus).
2. Important information before using Decapeptyl Depot
When not to use Decapeptyl Depot
if you are allergic to triptorelin or any of the other ingredients of this medicine (listed in section 6),
if you are allergic to gonadotropin-releasing hormone (GnRH) or any other GnRH analogue.
In women:
if you are pregnant,
if you are breastfeeding.
Before starting treatment with Decapeptyl 0.1 mg, discuss it with your doctor.
In men and women
There have been reports of depression in patients taking Decapeptyl Depot, including severe depression. If depressive symptoms occur during treatment with Decapeptyl Depot, inform your doctor.
In men
Caution should be exercised:
if you have bone pain or difficulty urinating,
if you have metastases in the spine or urinary tract,
if you have any cardiovascular disorders, including heart rhythm disorders (arrhythmia), or if you are taking medications for these disorders; the risk of heart rhythm disorders may be increased during treatment with Decapeptyl Depot.
Inform your doctor if any symptoms of the disease worsen.
In women
Caution should be exercised:
if you experience bleeding between menstrual periods (except for the first month),
if you have an increased risk of bone density loss.
Treatment with Decapeptyl Depot for several months may lead to a decrease in bone mass. Therefore, the treatment period should not exceed 6 months. After discontinuation of treatment, bone mass usually recovers within 6 to 9 months. Particular attention should be paid to patients with additional risk factors for osteoporosis.
During treatment
In the first month of treatment, a non-hormonal method of contraception should be used. It should also be used from the 4th week after the last injection until menstrual bleeding resumes or another method of contraception is used.
During treatment, menstrual bleeding does not occur. After treatment is stopped, menstrual bleeding usually resumes within 7-12 weeks after the last injection. If regular menstrual bleeding persists during treatment, inform your doctor.
Decapeptyl Depot and other medicines
Tell your doctor about all medicines you are taking or have recently taken, as well as any medicines you plan to take.
Decapeptyl Depot may interact with certain medicines used to treat heart rhythm disorders (e.g., quinidine, procainamide, amiodarone, and sotalol) or may increase the risk of heart rhythm disorders when used concomitantly with certain other medicines [e.g., methadone (used to reduce pain and as part of drug detoxification), moxifloxacin (an antibiotic), antipsychotic medicines used to treat severe mental disorders].
Caution should be exercised when triptorelin is administered concomitantly with medicines that affect gonadotropin secretion by the pituitary gland.
Pregnancy and breastfeeding
Decapeptyl Depot should not be used during pregnancy and breastfeeding (see also "When not to use Decapeptyl Depot").
Women of childbearing age should use effective, non-hormonal methods of contraception.
Page 2 of 9
Driving and using machines
Ability to drive and use machines may be impaired if dizziness, drowsiness, and vision disturbances occur, which may be side effects during treatment or result from the underlying disease.
Decapeptyl Depot contains sodium
Decapeptyl Depot contains less than 1 mmol of sodium (23 mg) per dose, which means that the medicine is considered "sodium-free".
3. How to use Decapeptyl Depot
Usually, mixing the powder and solvent and injecting are performed by medical personnel.
In men:
A dose contained in one prefilled syringe, equivalent to 3.75 mg of triptorelin, should be injected every 4 weeks. The injections should be given at 4-week intervals.
Prostate cancer:
Treatment is long-term.
Diagnosis of hormone-dependent prostate cancer:
Usually, treatment for 3 months is sufficient to determine hormone dependence of prostate cancer.
In women:
A dose contained in one prefilled syringe, equivalent to 3.75 mg of triptorelin, should be injected every 4 weeks. Treatment must be initiated within the first 5 days of the menstrual cycle.
The duration of treatment is supervised by a doctor.
Overdose of Decapeptyl Depot
It is unlikely that a patient will receive a higher dose of Decapeptyl Depot than intended. If a patient has been given a higher dose of Decapeptyl Depot than intended, inform a doctor immediately.
Discontinuation of Decapeptyl Depot
Discontinuation of treatment with Decapeptyl Depot should only be done under medical supervision. In case of doubts about the use of the medicine, consult a doctor.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
General (all patients):
If you experience swelling of the face, lips, tongue, or throat, which may cause difficulty swallowing or breathing, inform a doctor or go to the emergency department of the nearest hospital immediately.
During treatment with GnRH agonists, cases of enlargement of pre-existing pituitary tumors have been reported, but so far, no such cases have been observed in patients treated with triptorelin.
In men:
Page 3 of 9
Symptoms for which you are being treated (e.g., urinary obstruction, bone pain, spinal cord compression, muscle weakness, swelling of the lower limbs, weakness, tingling of the feet and hands) may initially worsen due to the initial, transient increase in testosterone levels at the start of treatment.
Very common– occurs in more than 1 in 10 patients:
most side effects of Decapeptyl Depot in men result from the decreased testosterone level; may occur:
impotence (erectile dysfunction),
decreased libido,
hot flashes,
excessive sweating.
Common– occurs in 1 to 10 patients in 100:
dizziness,
increased blood pressure,
bone pain,
fatigue,
pain at the injection site.
Uncommon– occurs in 1 to 10 patients in 1,000:
decreased appetite,
insomnia,
paresthesia (tingling, prickling, or numbness),
nausea,
constipation,
dry mouth,
hair loss,
back pain,
gynecomastia.
Frequency not known- cannot be estimated from the available data:
rhinitis (common cold),
dyspnea (shortness of breath) when lying down,
anaphylactic reactions,
hypersensitivity,
epistaxis (nosebleed),
increased appetite,
abdominal pain,
diabetes,
vomiting,
depression,
abdominal distension,
mood changes,
bloating,
confusion,
acne,
decreased activity,
pruritus (itching),
euphoric mood,
rash,
restlessness,
vesicles (small blisters),
apathy,
angioedema (swelling under the skin),
headache,
memory disorders,
urticaria (hives),
taste disorders,
purpura (purple spots on the skin),
drowsiness,
musculoskeletal pain,
difficulty standing,
limb pain,
abnormal sensation in the eye,
joint pain,
visual disturbances,
muscle spasms,
blurred vision,
muscle weakness,
tinnitus (ringing in the ears),
muscle pain,
balance disorders,
stiffness of the joints,
decreased blood pressure,
joint swelling,
dyspnea (shortness of breath),
musculoskeletal stiffness,
Page 4 of 9
osteochondritis (inflammation of bone and cartilage),
flu-like symptoms,
chest pain,
fever,
testicular atrophy,
malaise,
testicular pain,
changes in ECG (prolonged QT interval),
absence of ejaculation,
irritability,
weakness,
increased activity of certain liver enzymes,
redness at the injection site,
inflammation at the injection site,
increased blood creatinine levels,
reaction at the injection site,
increased blood urea levels,
edema,
increased blood pressure,
pain,
increased body temperature,
chills,
increased body weight,
decreased body weight.
In women:
In the initial period of treatment, symptoms of endometriosis, including pelvic pain and painful menstruation, may very often worsen due to the initial, transient increase in estradiol levels in the blood. These symptoms are temporary and usually resolve within one to two weeks.
Very common– occurs in more than 1 in 10 patients:
breast pain,
decreased libido,
sleep disorders,
hot flashes,
excessive sweating,
vaginal bleeding or spotting,
vaginal dryness,
headache.
Common– occurs in 1 to 10 patients in 100:
hyperandrogenism,
mood changes,
restlessness,
insomnia,
depression,
low mood,
dizziness,
palpitations,
respiratory symptoms,
nausea,
gastrointestinal disorders,
abdominal pain,
hair loss,
joint pain,
painful intercourse,
breast disorders,
fatigue,
weakness,
irritability,
weight gain,
abnormal weight.
Uncommon– occurs in 1 to 10 patients in 1,000:
paresthesia (tingling, prickling, or numbness),
balance disorders,
back pain,
bone pain,
Page 5 of 9
muscle spasms,
edema,
pain at the injection site,
reaction at the injection site,
increased blood pressure.
Rare– occurs in 1 to 10 patients in 10,000:
vomiting,
muscle pain,
excessive menstrual bleeding,
pelvic pain.
Frequency not known- cannot be estimated from the available data:
hypersensitivity,
pruritus (itching)
anaphylactic reactions,
rash
abdominal symptoms
urticaria (hives)
fever
malaise
painful menstruation,
diarrhea
bleeding between normal menstrual periods,
muscle weakness
blurred vision
angioedema (swelling under the skin),
visual disturbances,
dyspnea (shortness of breath),
amenorrhea (absence of menstruation)
confusion,
breast pain,
inflammation at the injection site,
redness at the injection site
Reporting side effects
If you experience any side effects, including those not listed in this package leaflet, inform your doctor. Side effects can be reported directly to the Department of Drug Safety Monitoring of the Office for Registration of Medicinal Products, Medical Devices, and Biocidal Products: Aleje Jerozolimskie 181C, 02-222 Warsaw, Tel: (22) 49-21-301, Fax: (22) 49-21-309 or at https://smz.ezdrowie.gov.pl/.
Side effects can also be reported to the marketing authorization holder.
By reporting side effects, you can help provide more information on the safety of this medicine.
5. How to store Decapeptyl Depot
Keep the medicine out of the sight and reach of children.
Do not use this medicine after the expiry date stated on the packaging after EXP.
The expiry date refers to the last day of the month stated.
Store in a refrigerator (2°C – 8°C). Store in the original packaging.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. This will help protect the environment.
6. Contents of the pack and other information
What Decapeptyl Depot contains
- The active substance is triptorelin in a quantity of 3.75 mg
- Other ingredients are: Powder: poly(lactic acid and glycolic acid) 1:1 and propylene glycol dicaprylate/caprate
Page 6 of 9
Solvent: Polysorbate 80, Dextran 70, sodium chloride, sodium dihydrogen phosphate dihydrate, sodium hydroxide, and water for injections.
What Decapeptyl Depot looks like and contents of the pack
Decapeptyl Depot is a powder and solvent for suspension for injection.
The powder is white to slightly yellowish.
The solvent is a clear, colorless liquid.
After mixing the powder with the solvent, a homogeneous, milky white to slightly yellowish suspension should be obtained.
One pack size is available: 1 prefilled syringe with powder, 1 prefilled syringe with solvent, 1 connector made of polypropylene, and 1 injection needle, all in a cardboard box.
Marketing authorization holder and manufacturer:
Ferring GmbH
Wittland 11
D-24109 Kiel
Germany
For more information, contact the representative of the marketing authorization holder:
Ferring Pharmaceuticals Poland Sp. z o.o.
Szamocka 8, 01-748 Warsaw
Tel.: +48 22 246 06 80, Fax: +48 22 246 06 81
Date of last revision of the package leaflet: -------------------------------------------------------------------------------------------------------------------------------------
Information intended for healthcare professionals only:
Instructions for the doctor on preparing, mixing, and injecting the suspension.
Since proper preparation of the suspension is crucial for the therapeutic effectiveness of the product, the following instructions must be strictly followed:

Important information:
DECAPEPTYL Depot should be stored in the refrigerator.
DECAPEPTYL Depot should be injected within 3 minutes of preparation.
Review of the components of the DECAPEPTYL Depot kit:



Page 7 of 9
1. Preparation
To ensure proper preparation of the suspension, follow the instructions exactly:
Remove the DECAPEPTYL Depot packaging from the refrigerator.
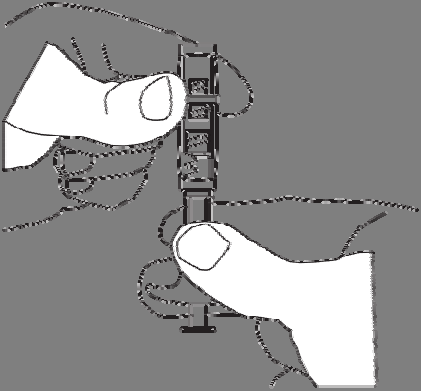
Open the connector packaging and remove the connector.
Remove the cap from the syringe with the powder. Hold the syringe with the opening facing upwards to avoid spilling the powder.
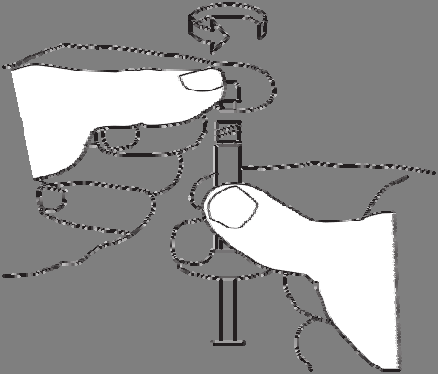
Screw the syringe with the powder onto one of the threads of the connector until it stops.



Always connect the syringe containing the powder to the
connector first, and then the syringe containing the
solvent.
Remove the cap from the syringe with the solvent.
Hold the syringe with the opening facing upwards to avoid spilling the liquid.
Do not touch the connector threads.
Do not press the syringe plunger.
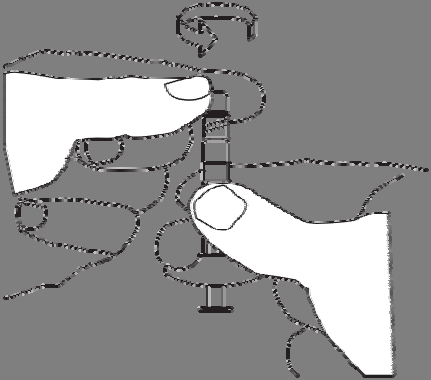







Screw the syringe containing the solvent onto the free thread of the connector until it stops.

Do not press the syringe plunger.
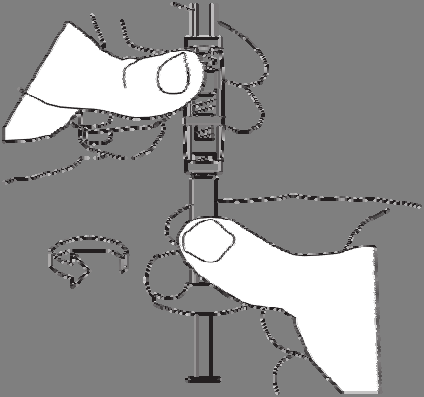


2. Preparation of the suspension
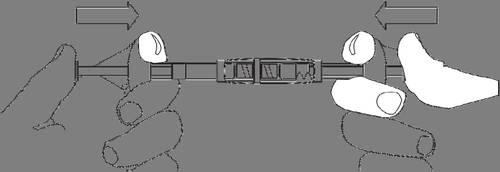
To prepare the suspension, follow these steps:
Inject the liquid into the syringe with the powder.
Slowly push the suspension back and forth between the two syringes until a homogeneous, milky white to slightly yellowish suspension is obtained.
Hold the syringes straight, do not bend them.
Page 8 of 9
3. Injection
Remove the syringe with the prepared suspension from the connector.
Attach the injection needle to the syringe.
Perform the injection within 3 minutes.
DECAPEPTYL Depot is for single use only, and any unused suspension should be discarded.
Page 9 of 9
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- ImporterFerring GmbH
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to Decapeptil DepotDosage form: Solution, 0.1 mg/mlActive substance: triptorelinManufacturer: Ferring GmbHPrescription requiredDosage form: Powder, 0.1 mgActive substance: triptorelinManufacturer: Ipsen Pharma Biotech SASPrescription requiredDosage form: Powder, 11.25 mgActive substance: triptorelinManufacturer: Ipsen Pharma Biotech SASPrescription required
Alternatives to Decapeptil Depot in other countries
The best alternatives with the same active ingredient and therapeutic effect.
Alternative to Decapeptil Depot in Ukraine
Alternative to Decapeptil Depot in Spain
Online doctors for Decapeptil Depot
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for Decapeptil Depot – subject to medical assessment and local rules.











