DECAPEPTYL MENSUAL 3.75 mg POWDER AND SOLVENT FOR PROLONGED-RELEASE INJECTABLE SUSPENSION


How to use DECAPEPTYL MENSUAL 3.75 mg POWDER AND SOLVENT FOR PROLONGED-RELEASE INJECTABLE SUSPENSION
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
- Introduction
- What is Decapeptyl Monthly and what is it used for
- What you need to know before you use Decapeptyl Monthly
- If you are using Decapeptyl Monthly for the adjuvant treatment of breast cancer:
- How to use Decapeptyl Monthly
- Possible Adverse Effects
- Storage of Decapeptyl Monthly
- Container Content and Additional Information
Introduction
Package Leaflet: Information for the User
Decapeptyl Monthly 3.75 mg Powder and Solvent for Prolonged-Release Suspension for Injection
Triptorelin
Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the Package Leaflet
- What is Decapeptyl Monthly and what is it used for.
- What you need to know before you use Decapeptyl Monthly.
- How to use Decapeptyl Monthly.
- Possible side effects.
- Storing Decapeptyl Monthly.
- Package Contents and Additional Information
1. What is Decapeptyl Monthly and what is it used for
This medicine contains triptorelin. Triptorelin belongs to a group of medicines known as gonadotropin-releasing hormone (GnRH) analogues. One of its actions is to decrease the levels of sex hormones in the body.
Decapeptyl Monthly is indicated in adults for the treatment of hormone-dependent prostate cancer that is locally advanced or has spread to other parts of the body (metastatic cancer). It is also used to treat localized prostate cancer that is at high risk or locally advanced, in combination with radiotherapy.
Decapeptyl Monthly is also indicated in women for the treatment of endometriosis, uterine fibroids, female infertility, and early-stage hormone-sensitive breast cancer, in premenopausal women who have received chemotherapy, and in children, for central precocious puberty.
In breast cancer, Decapeptyl Monthly is used in conjunction with other hormonal medicines:
- A medicine called tamoxifen (this medicine will be prescribed for you if you are at high risk of cancer recurrence) or,
- An "aromatase inhibitor" such as exemestane (you will be prescribed Decapeptyl Monthly for at least 6 to 8 weeks before you start taking this medicine).
Remember to read the package leaflet of the medicine you are taking with Decapeptyl Monthly.
2. What you need to know before you use Decapeptyl Monthly
Do not use Decapeptyl Monthly:
- if you are allergic (hypersensitive) to triptorelin, to gonadotropin-releasing hormone (GnRH), to other GnRH analogues, or to any of the other ingredients of this medicine (listed in section 6).
- if you are pregnant or breastfeeding.
- If you are taking Decapeptyl Monthly for breast cancer, do not use an "aromatase inhibitor" (such as exemestane) until you have been treated with Decapeptyl Monthly for at least 6 to 8 weeks.
Warnings and Precautions:
Consult your doctor or pharmacist before starting treatment with Decapeptyl Monthly:
Depression has been reported in patients treated with Decapeptyl Monthly, which can be severe. If you are being treated with Decapeptyl Monthly and experience depression, inform your doctor. Your doctor may want to monitor your depression during treatment.
In adults, triptorelin may cause bones to become less dense (osteoporosis) with an increased risk of bone fractures. Therefore, inform your doctor if you have any risk factors, as your doctor may prescribe a bisphosphonate (a medicine used to treat weak bones) to treat bone loss. Risk factors may include:
- If you or a close family member has osteoporosis.
- If you drink excessive amounts of alcohol and/or are a heavy smoker.
If you have been taking medicines that can cause your bones to become less dense, such as medicines for epilepsy or steroids (such as hydrocortisone or prednisolone).
If you experience seizures, inform your doctor immediately. Seizures have been reported in patients receiving triptorelin or similar medicines. These occurred in patients with or without a medical history of epilepsy.
If you have an enlarged pituitary gland (a benign tumor) that you were not aware of, this may be discovered during treatment with Decapeptyl Monthly. Symptoms include headache, vision problems, and paralysis of the eyes.
If you are taking medicines to prevent blood clotting, as bruising may occur at the injection site.
If you have diabetes or heart problems, inform your doctor.
Men
- When starting treatment, the amount of testosterone in your body will increase, which can make cancer symptoms worse. Consult your doctor if this happens. Your doctor may give you a medicine (an anti-androgen) to prevent symptoms from getting worse.
- If you have urinary obstruction or spinal cord compression (nerves in your spine) due to cancer spread, during the first few weeks of treatment, your doctor will closely monitor you. If you experience difficulty urinating, bone pain, weakness in your legs, or numbness, consult your doctor immediately, who will evaluate and treat you accordingly.
- After surgical castration, triptorelin does not induce any further decrease in serum testosterone levels and therefore should not be used after orchiectomy (surgical removal of the testicles).
- Diagnostic tests for pituitary-gonadal function or sexual organs performed during or after treatment with Decapeptyl Monthly may be misleading.
- If you have any problems with your blood vessels or heart, including heart rhythm problems (arrhythmia) or if you are being treated with medicines for this condition, consult your doctor. The risk of heart rhythm problems may be increased with Decapeptyl Monthly.
- Medicines that decrease testosterone may cause changes in the ECG associated with heart rhythm abnormalities (prolongation of the QT interval).
- Treatment with GnRH analogues like Decapeptyl Monthly may increase the risk of anemia (defined as a decrease in red blood cell count).
Women
Due to the lack of clinical experience in women under 18 years of age, the use of triptorelin is not recommended in adolescents and young women as it may cause a decrease in bone density.
During the first month of treatment, you may experience some vaginal bleeding. After that, your menstruation will stop. Inform your doctor if you continue to experience bleeding after the first month. After the last injection, you will have your menstruation back after about 2 months. You should use a non-hormonal contraceptive method during the first month of treatment and after the last injection, unless the treatment is for infertility.
If you have submucous uterine fibroids (benign tumors in the muscle under the uterine lining), triptorelin may cause bleeding when the fibroids break down within the first 6-10 weeks after starting treatment. Contact your doctor immediately if you experience bleeding or severe and unusual pain.
During infertility treatment, gonadotropins (hormones that stimulate the ovaries) combined with this medicine may cause an increase in ovarian size or ovarian hyperstimulation, which can cause pelvic and/or abdominal pain and breathing difficulties. If this occurs, consult your doctor immediately.
If you are using Decapeptyl Monthly for the adjuvant treatment of breast cancer:
- If you have any problems with your bones, such as osteoporosis, inform your doctor. This may affect how your doctor decides to treat you. Your doctor will perform a bone scan before you start treatment if you are at risk of osteoporosis and will monitor you during treatment.
- If you have diabetes or high blood pressure, inform your doctor. Your doctor will monitor your blood sugar and blood pressure levels during treatment.
- If you have depression, inform your doctor. Your doctor may want to monitor your depression during treatment.
- If you stop treatment with triptorelin, you must also stop treatment with the aromatase inhibitor (such as exemestane) at the same time.
Children
Girls with central precocious puberty may experience some vaginal bleeding during the first month of treatment.
If you have a progressive brain tumor, inform your doctor. This may change how your doctor decides to treat you.
Your doctor must rule out precocious puberty caused by other diseases.
The amount of minerals in the bones decreases during treatment but returns to normal levels after stopping treatment.
After stopping treatment, a hip disorder (slipped capital femoral epiphysis) may occur. This causes stiffness in the hip, limping, and/or severe pain in the groin that radiates to the thigh. If this occurs, you should consult your doctor.
If your child experiences severe or recurrent headaches, vision problems, or ringing in the ears, contact a doctor immediately (see section 4).
Consult your doctor if you are concerned about any of these issues.
Other medicines and Decapeptyl Monthly
Tell your doctor or pharmacist if you are using, or have recently used, other medicines, including those obtained without a prescription.
For men
Decapeptyl Monthly may interfere with some medicines used to treat heart rhythm problems (e.g., quinidine, procainamide, amiodarone, and sotalol) or may increase the risk of heart rhythm problems when used with other medicines (e.g., methadone (used for pain relief and as part of drug detoxification), moxifloxacin (an antibiotic), antipsychotics used for severe mental illnesses).
Pregnancy and Breastfeeding
Decapeptyl Monthly must not be used during pregnancy or breastfeeding.
Do not use Decapeptyl if you are trying to become pregnant (unless Decapeptyl is part of a treatment for infertility).
Driving and Using Machines
You may feel dizzy, tired, or have vision problems, such as blurred vision. These are possible side effects of treatment or due to the underlying disease. If you experience any of these side effects, do not drive or use machines.
Decapeptyl Monthly 3.75 mg contains sodium
This medicine contains sodium, but less than 1 mmol (23 mg) of sodium per vial; this is essentially "sodium-free".
3. How to use Decapeptyl Monthly
Follow exactly the administration instructions of this medicine given by your doctor. If you are unsure, consult your doctor or pharmacist again.
Decapeptyl Monthly must be administered exclusively by the intramuscular route. Your doctor or nurse will administer it. See the instructions for use at the end of this leaflet.
For localized prostate cancer that is at high risk or locally advanced, in combination with radiotherapy, the recommended treatment duration is 2-3 years.
The dose will be determined by your doctor based on the needs of each patient. The usual doses are as follows:
Prostate cancer: One deep intramuscular injection of Decapeptyl Monthly every four weeks.
Endometriosis: Treatment should start during the first five days of the cycle. One deep intramuscular injection of Decapeptyl Monthly every four weeks.
The duration of treatment depends on the initial severity of endometriosis and the evolution of clinical manifestations (functional and anatomical) during treatment. In principle, the duration of treatment should be four to six months. A second treatment with Decapeptyl Monthly or another medicine of the same group is not recommended.
Uterine fibroids: Treatment should start during the first five days of the cycle. The recommended dose is one deep intramuscular injection every four weeks. The duration of treatment depends on the evolution of fibroid volume, determined by ultrasound. In principle, fibroids should be treated for no more than six months (see adverse reactions). A second treatment with Decapeptyl Monthly or another medicine of the same group is not recommended.
Female infertility: One deep intramuscular injection of Decapeptyl Monthly administered on the second day of the cycle. In general, stimulation with this medicine should be performed when plasma estrogen levels are below 50 pg/ml (usually around day 15 of the cycle).
Breast cancer
The recommended dose of Decapeptyl Monthly is one intramuscular injection every 4 weeks (28 days). Treatment may last up to 5 years.
Decapeptyl Monthly is used in conjunction with a medicine called tamoxifen or an "aromatase inhibitor", such as exemestane. If you need to take an "aromatase inhibitor", you should be treated with Decapeptyl Monthly for at least 6 to 8 weeks before you start taking it.
Central precocious puberty:
In children: the dose is adjusted according to body weight.
Children over 30 kg in weight: one intramuscular injection every 4 weeks (28 days).
Children between 20 and 30 kg in weight: two-thirds of the dose by intramuscular injection every 4 weeks (28 days), i.e., administer two-thirds of the volume of the reconstituted suspension.
Children under 20 kg in weight: half a dose by intramuscular injection every 4 weeks (28 days), i.e., administer half of the volume of the reconstituted suspension.
If you use more Decapeptyl Monthly than you should
In case of overdose or accidental ingestion, consult your doctor or pharmacist immediately.
If you forget to use Decapeptyl Monthly
As soon as you remember that you have missed an injection, consult your doctor, who will decide when you should have your next injection.
If you stop treatment with Decapeptyl Monthly
Do not stop treatment with Decapeptyl Monthly without talking to your doctor first. This is especially important if you are using Decapeptyl Monthly with an aromatase inhibitor. This is because stopping treatment may cause an increase in estrogen levels. Your doctor will monitor your estrogen levels during treatment with Decapeptyl Monthly.
If you stop treatment with Decapeptyl Monthly, you must also stop treatment with the aromatase inhibitor within 1 month of stopping treatment.
4. Possible Adverse Effects
Like all medicines, this medicine can cause adverse effects, although not all people suffer from them.
In rare cases, you may experience a severe allergic reaction (angioedema, anaphylactic reaction). Inform your doctor immediately if you develop symptoms such as difficulty swallowing or breathing, dizziness, swelling of the lips, face, throat, or tongue, or a rash.
If you have an enlargement (benign tumor) of the pituitary gland that you were unaware of, it may be discovered during treatment with Decapeptyl monthly. The symptoms include sudden headache, vision problems, and paralysis of the eyes.
As with other GnRH analogs, an increase in the white blood cell count may occur in patients treated with Decapeptyl monthly.
Men
Many of these adverse effects are expected due to the change in testosterone levels in your body. These effects include hot flashes, impotence, and decreased libido.
Very frequent adverse effects, which may affect more than 1 in 10 patients:
- Hot flashes
- Weakness
- Excessive sweating
- Back pain
- Feeling of tingling and numbness in the legs
- Decreased libido
- Impotence
Frequent adverse effects, which may affect 1 to 10 out of 100 patients:
- Nausea, dry mouth
- Pain, bruising, redness, and swelling at the injection site, muscle and bone pain, pain in arms and legs, edema (fluid accumulation in body tissues), lower abdominal pain
- Allergic reaction
- Weight gain
- Dizziness, headache
- Loss of libido, depression, mood changes
- High blood pressure
Infrequent adverse effects, which may affect 1 to 10 out of 1,000 patients:
- Increased platelet count
- Feeling of heartbeats
- Ringing in the ears, vertigo
- Blurred vision
- Abdominal pain, constipation, diarrhea, vomiting
- Drowsiness, intense shivering associated with sweating and fever, somnolence, pain
- Certain analytical parameters affected (including increased liver function tests), increased blood pressure
- Weight loss
- Loss of appetite, increased appetite, gout (severe pain and swelling of the joints, usually in the big toe), diabetes, excess lipids in the blood
- Joint pain, muscle cramps, muscle weakness, muscle pain, swelling of the ankles, feet, or fingers, bone pain
- Tingling or numbness
- Insomnia, feeling of irritability
- Waking up to urinate, difficulty urinating
- Development of breast tissue in men, chest pain, reduced testicular size, testicular pain
- Difficulty breathing
- Nosebleeds
- Acne, hair loss, itching, rash, skin redness, hives
Rare adverse effects, which may affect 1 to 10 out of 10,000 patients:
- Red or purple discoloration of the skin
- Abnormal sensation in the eye, altered vision, or blurred vision
- Feeling of full abdomen, flatulence, abnormal taste
- Chest pain
- Difficulty standing
- Flu-like symptoms, fever
- Severe allergic reaction that can cause dizziness or difficulty breathing, swelling of the face or throat
- Nasal/throat inflammation
- Increased body temperature
- Joint stiffness, joint swelling, musculoskeletal stiffness, osteoarthritis (joint disorder that affects cartilage and causes pain, swelling, and loss of movement)
- Memory loss
- Feeling of confusion, decreased activity, feeling of euphoria
- Difficulty breathing when lying down
- Blisters
- Low blood pressure
During post-marketing surveillance, the following adverse effects have also been reported:
- Severe allergic reaction that can cause inflammation of the face, tongue, and throat, difficulty breathing or dizziness (Quincke's edema, anaphylactic shock)
- Changes in the ECG (QT prolongation)
- General malaise
- Anxiety
- Rapid formation of papules caused by swelling of the skin or mucous membranes
- Urinary incontinence
- If there is a pre-existing pituitary tumor, there is an increased risk of bleeding in the area
- Anemia (decrease in red blood cell count)
Patients who are receiving long-term treatment with GnRH analogs in combination with radiation may have more adverse effects, especially gastrointestinal ones, related to radiotherapy.
Women:
Many of the adverse effects are expected due to the change in estrogen levels in the body.
Very frequent adverse effects (affect more than 1 patient in 10):
- Mood changes, difficulty sleeping
- Headache
- Decreased libido
- Hot flashes
- Excessive sweating, acne, oily skin
- Breast disorders
- Pain during or after sexual intercourse, painful menstruation, genital bleeding
- Ovarian hyperstimulation syndrome (with increased ovarian size and fluid retention)
- Pelvic pain, enlarged ovaries
- Vaginal dryness
- Weakness
Frequent adverse effects (affect 1 to 10 patients out of 100):
- Allergic reaction
- Depression (long-term treatment), nervousness
- Dizziness
- Weight gain, nausea, abdominal pain or discomfort
- Chest pain
- Muscle cramps, painful joints
- Pain in arms and legs
- Reaction at the injection site (including pain, swelling, redness, and inflammation)
- Swelling of ankles, feet, and fingers
Infrequent adverse effects (affect 1 to 10 patients out of 1,000):
- Weight loss, decreased appetite
- Fluid retention
- Mood changes, anxiety, disorientation, depression (short-term treatment)
- Abnormal taste
- Loss of sensation
- Temporary loss of consciousness, memory loss, lack of concentration
- Tingling or numbness, involuntary muscle movement
- Dry eyes, blurred vision
- Vertigo
- Feeling of heartbeats
- Difficulty breathing, nosebleeds
- Abdominal swelling, vomiting, dry mouth, flatulence, mouth ulcers
- Hair loss, excess body hair
- Dry skin, brittle nails, itching, skin rash
- Back pain, muscle pain
- Bleeding after sexual intercourse, prolapse, irregular menstruation, painful and intense menstruation
- Small cysts (inflammation) in the ovaries that can cause pain, vaginal discharge
During post-marketing experience, the following adverse effects have also been reported:
- Severe allergic reaction that can cause inflammation of the face, tongue, and throat, difficulty breathing or dizziness (Quincke's edema, anaphylactic shock)
- Seizures
- Confusion, abnormal sensations in the eyes and/or changes in vision
- Urticaria (rapid formation of papules due to inflammation of the skin or mucous membranes)
- Muscle weakness
- Absence of menstrual periods
- Fever and general malaise
- Affectation of some blood tests (including elevated liver function tests)
- Diarrhea
- Increased blood pressure
- If there is a pre-existing pituitary tumor, there is an increased risk of bleeding in the area
During infertility treatment, the gonadotropins combined with the product may induce an increase in ovarian size or ovarian hyperstimulation, which can cause pelvic or abdominal pain or difficulty breathing. If this occurs, consult your doctor immediately.
In the treatment of endometriosis, the disorders for which treatment has been justified (pelvic pain, dysmenorrhea) may worsen at the beginning of treatment, but they should disappear within one or two weeks. This may occur even if the treatment has a favorable effect. However, you should inform your doctor immediately of this phenomenon.
Adverse Effects when Used for Breast Cancer in Combination with Tamoxifen or an Aromatase Inhibitor:
The following adverse effects have been observed when triptorelin is used for breast cancer in combination with tamoxifen or an aromatase inhibitor.
Very frequent adverse effects (affect more than 1 patient in 10):
- Nausea
- Feeling very tired
- Joint and muscle pain, osteoporosis
- Hot flashes
- Excessive sweating
- Difficulty sleeping, depression
- Decreased libido, vaginal dryness, pain during or after sexual intercourse
- Urinary incontinence
- Increased blood pressure
Frequent adverse effects (affect 1 to 10 patients out of 100):
- Diabetes, elevated blood sugar (hyperglycemia)
- Pain, bruising
- Redness and inflammation at the injection site
- Allergic reaction
- Bone fractures
- Blood clots in a blood vessel
Infrequent adverse effects (affect 1 to 10 patients out of 1,000):
- Bleeding in the brain
- Lack of blood supply to the brain or heart
Rare adverse effects (affect 1 to 10 patients out of 10,000):
- Change in the ECG (QT prolongation)
Children
Very frequent adverse effects (affect more than 1 patient in 10):
- Vaginal bleeding, which may occur during the first month, in girls
Frequent adverse effects (affect 1 to 10 patients out of 100):
- Abdominal pain
- Painful bruising
- Redness and inflammation at the injection site
- Headache, hot flashes
- Weight gain
- Acne
- Allergic reaction
Infrequent adverse effects (affect 1 to 10 patients out of 1,000):
- Blurred vision
- Vomiting, constipation, nausea
- General malaise
- Overweight
- Neck pain, chest pain
- Mood changes
- Nosebleeds
- Itching, skin rash, or urticaria
During post-marketing experience, the following adverse effects have also been reported:
- Severe allergic reaction that can cause difficulty breathing or dizziness and inflammation of the face, neck, or throat (Quincke's edema, anaphylactic shock)
- Seizures
- High blood pressure
- Abnormal vision
- Some blood tests affected, including hormonal levels
- Muscle pain
- Mood changes, depression, nervousness
- Idiopathic intracranial hypertension (increased pressure around the brain, characterized by headache, double vision, and other visual symptoms, and ringing or buzzing in the ears)
Reporting of Adverse Effects
If you experience any type of adverse effect, consult your doctor, even if it is a possible adverse effect that is not listed in this prospectus. You can also report it directly through the Spanish Pharmacovigilance System for Human Use Medicines. www.notificaRAM.es. By reporting adverse effects, you can contribute to providing more information on the safety of this medicine.
5. Storage of Decapeptyl Monthly
Keep this medicine out of the sight and reach of children.
Store Decapeptyl monthly in its original packaging. Do not store at a temperature above 25°C.
Do not use Decapeptyl monthly after the expiration date that appears on the packaging after CAD. The expiration date is the last day of the month indicated.
Medicines should not be disposed of through wastewater or household waste. Deposit the packaging and medicines you no longer need at the SIGRE point in the pharmacy. In case of doubt, ask your pharmacist how to dispose of the packaging and medicines you no longer need. This will help protect the environment.
6. Container Content and Additional Information
Composition of Decapeptyl Monthly
- The active ingredient is triptorelin (acetate), 3.75 mg per vial.
The other components are:
- Powder: DL lactide-co-glycolide polymer, mannitol, sodium carmellose, polysorbate 80
- Solvent: mannitol and water for injectable preparations.
Appearance of the Product and Container Content
This medication is a powder and solvent for injectable suspension, the powder is a lyophilized powder of a whitish color and the solvent for the reconstitution of the suspension is a clear, colorless solution.
Container with 1 vial, 1 ampoule, and 1 blister pack with 1 syringe and 2 needles for reconstitution and administration.
Marketing Authorization Holder and Manufacturer
Marketing Authorization Holder:
IPSEN PHARMA, S.A.U.
Gran Via de les Corts Catalanes 130-136
08038 Barcelona
Spain
Manufacturer:
IPSEN PHARMA-BIOTECH
Parc d'Activité du Plateau de Signes, C.D. 402,
83870 Signes
France
Date of the Last Revision of this Prospectus: February 2025
Detailed and updated information on this medication is available on the website of the Spanish Agency for Medicines and Health Products (AEMPS) http://www.aemps.gob.es
This information is intended solely for healthcare professionals:
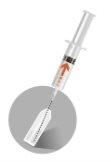
INSTRUCTIONS FOR RECONSTITUTION
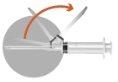
1 –PATIENT PREPARATION BEFORE RECONSTITUTION | |
Prepare the patient by disinfecting the injection site on the buttock. This operation must be performed first, as once reconstituted, the product must be injected immediately. | |
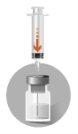
2 – PREPARATION OF THE INJECTION | |
The box includes two needles:
| |
The presence of bubbles in the upper part of the lyophilized powder is a normal aspect of the product. The following steps must be completed in a continuous sequence. | |
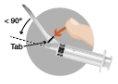
2a
|
|
2b
|
|
2c
|
|
2d
|
|
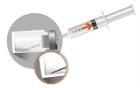
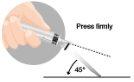
3 –INTRAMUSCULAR INJECTION | |
|
|
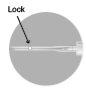
4 –AFTER USE | |
There are two alternative methods to activate the safety system.
Used needles, any unused suspension, or other residual material must be discarded in accordance with local guidelines. |
Method A
Method B
|
Any incident that causes significant loss of the product to be injected must be reported to the doctor, who will determine the need to repeat the injection at a shorter interval.
|
TREATMENT TABLE
SURNAMES
NAME
Request Date | Injection Date | |
Injection 1 | ||
Injection 2 | ||
Injection 3 | ||
Injection 4 | ||
Injection 5 | ||
Injection 6 |
- Country of registration
- Average pharmacy price127.95 EUR
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to DECAPEPTYL MENSUAL 3.75 mg POWDER AND SOLVENT FOR PROLONGED-RELEASE INJECTABLE SUSPENSIONDosage form: INJECTABLE, 0.1 mgActive substance: triptorelinManufacturer: Ipsen Pharma S.A.Prescription requiredDosage form: INJECTABLE, 22.5 mgActive substance: triptorelinManufacturer: Ipsen Pharma S.A.U.Prescription requiredDosage form: INJECTABLE, 11.25 mg triptorelinActive substance: triptorelinManufacturer: Ipsen Pharma S.A.Prescription required
Online doctors for DECAPEPTYL MENSUAL 3.75 mg POWDER AND SOLVENT FOR PROLONGED-RELEASE INJECTABLE SUSPENSION
Discuss questions about DECAPEPTYL MENSUAL 3.75 mg POWDER AND SOLVENT FOR PROLONGED-RELEASE INJECTABLE SUSPENSION, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions