

Dexaprotect

Ask a doctor about a prescription for Dexaprotect

How to use Dexaprotect
Leaflet accompanying the packaging: information for the user
Dexaprotect, 1 mg/ml, eye drops, solution
Dexamethasone phosphate
Read the leaflet carefully before using the medicine, as it contains important information for the patient.
- Keep this leaflet, you may need to read it again.
- In case of any doubts, consult a doctor, pharmacist, or nurse.
- This medicine has been prescribed specifically for you. Do not pass it on to others. The medicine may harm another person, even if their symptoms are the same.
- If the patient experiences any side effects, including any side effects not listed in this leaflet, they should tell their doctor, pharmacist, or nurse. See section 4.
Table of contents of the leaflet
- 1. What is Dexaprotect and what is it used for
- 2. Important information before using Dexaprotect
- 3. How to use Dexaprotect
- 4. Possible side effects
- 5. How to store Dexaprotect
- 6. Contents of the packaging and other information
1. What is Dexaprotect and what is it used for
Dexaprotect contains dexamethasone, a corticosteroid used to suppress the symptoms of inflammatory conditions (such as pain, swelling with local warmth and redness).
Dexaprotect is used to treat inflammatory conditions of the eyes.
If the patient has an eye infection (redness of the eye, tearing, and discharge), the patient will receive another medicine to take at the same time as Dexaprotect. See section 2.
Dexaprotect eye drops, solution, is a sterile solution that does not contain preservatives.
2. Important information before using Dexaprotect
When not to use Dexaprotect
- if the patient is allergic to dexamethasone phosphate or any of the other ingredients of this medicine (listed in section 6),
- if the patient has an eye infection for which they are not taking any medicine,
- if the patient has damage to the surface of the eye (small holes (perforation), ulcers, or injuries that have not been properly healed),
- if the patient has high pressure in the eye, known to be caused by glucocorticoids (a family of medicines containing corticosteroids)
Warnings and precautions
Before starting to use Dexaprotect, discuss it with your doctor, pharmacist, or nurse.
- Do not inject, do not swallow.
- Avoid contact between the tip of the dispenser and the eye or eyelids.
- Close ophthalmological monitoring is necessary during the use of Dexaprotect, especially:
- in children and the elderly, more frequent monitoring (eye examination) is recommended.
- if the patient has an eye infection. Dexaprotect should only be used if the patient is also taking an anti-infective medicine.
- if the patient has a corneal ulcer, an open wound on the surface of the eye, sometimes with severe pain, tearing, squinting, and vision loss. Do not use Dexaprotect unless the inflammatory condition is the primary cause of delayed healing.
- if the patient has high pressure in the eye. If the patient has had high pressure in the eye after using a steroid eye medicine, there is a risk of high pressure in the eye when using Dexaprotect.
- if the patient has glaucoma, a condition that can cause damage to the optic nerve and vision loss.
- At the first sign of corneal calcification, the medicine should be discontinued, and the patient should be treated with a phosphate-free medicine.
- Children: do not use long-term, continuous treatment.
- If the patient has severe allergic conjunctivitis (redness, swelling, itching, and tearing in the eye) that another medicine has not been able to cure, Dexaprotect should only be used for a short time.
- Patients with diabetes: if the patient has diabetes, they should inform their ophthalmologist or optician.
- If the patient has a red eye whose cause has not been diagnosed, they should not use Dexaprotect.
- Contact lenses: do not wear contact lenses while using Dexaprotect.
Patient with a history of contact hypersensitivity to silver should not use this product.
Consult a doctor if the patient experiences swelling and weight gain around the trunk and face, as these are usually the first symptoms of Cushing's syndrome. After stopping long-term or intensive treatment with Dexaprotect, adrenal insufficiency may develop.
Consult a doctor before stopping treatment on your own. This risk is particularly important in children and patients treated with ritonavir or cobicistat.
If the patient experiences blurred vision or other vision disturbances, they should contact their doctor.
Dexaprotect and other medicines
Tell your doctor or pharmacist about all medicines the patient is taking or has recently taken, including medicines they plan to take.
If the patient is using another eye medicine, they should wait 15 minutes before administering the next medicine.
Concomitant use of eye drops containing steroid medicines and eye drops containing beta-adrenergic blocking medicines (for the treatment of high pressure in the eye) may cause calcium phosphate deposits on the surface of the eye.
Tell your doctor if the patient is taking ritonavir or cobicistat, as these medicines may increase the level of dexamethasone in the blood.
Pregnancy, breastfeeding, and fertility
If the patient is pregnant or breastfeeding, thinks they may be pregnant, or plans to have a child, they should consult their doctor or pharmacist before using this medicine.
There is not enough information about the use of dexamethasone during pregnancy to determine possible side effects. Therefore, it is not recommended to use Dexaprotect during pregnancy.
It is not known whether this medicine passes into breast milk. However, the dose of Dexaprotect used is small. Dexaprotect can be used during breastfeeding.
Driving and using machines
For a short time after using the drops, the patient may experience blurred vision. Before continuing to drive or operate machinery, they should wait until their vision is clear.
Dexaprotect contains phosphate buffer
This medicine contains 1.976 mg of phosphate per milliliter of solution, which corresponds to 7.450 mg of disodium phosphate dodecahydrate.
If the patient has severe damage to the transparent layer on the front of the eye (cornea), phosphates may rarely cause cloudy spots on the cornea, caused by calcium accumulation during treatment.
3. How to use Dexaprotect
This medicine should always be used as directed by the doctor. In case of doubts, consult a doctor or pharmacist.
The recommended dose is 1 drop administered 4 to 6 times a day to the affected eye. If the patient's condition is more serious, the doctor may recommend starting treatment with 1 drop every hour, and then, after the medicine starts to work, changing the dose to 1 drop every 4 hours. It is essential to gradually reduce the dose to avoid worsening the condition.
Elderly patients
No dose adjustment is necessary.
Use in children
Do not use this medicine for long-term, continuous treatment.
Method of administration
Administration to the eye: this medicine is in the form of eye drops to be administered to the eye (eyes).
Do not touch the eye or the area around the eye with the tip of the multidose container. This could cause eye injuries. It could also cause contamination of the eye drop solution with bacteria and lead to severe eye damage or even vision loss.
To avoid possible contamination of the multidose container, keep the tip of the container away from contact with any surface.
Before instilling the eye drops:
- Wash your hands before opening the bottle.
- Do not use this medicine if the protective seal on the neck of the bottle was broken before the first use of the medicine.
- When using the medicine for the first time, before instilling the eye drops, first practice using the bottle with the dropper by slowly squeezing the bottle to administer one drop in the air, away from the eye.
- Once the patient is sure they can administer one drop at a time, they should take the most comfortable position for instillation (they can sit, lie on their back, or stand in front of a mirror).
Instillation:
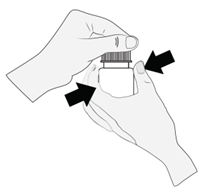
- 1. Hold the bottle just below the cap and unscrew it to open the bottle. Do not touch anything with the tip of the bottle to avoid contaminating the solution.
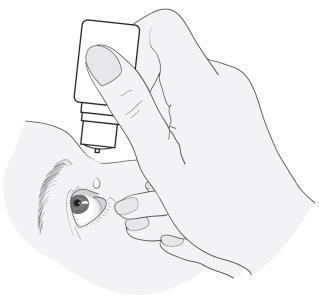
- 2. Tilt the head back and hold the bottle over the eye.
- 3. Pull the lower eyelid down and look up. Gently squeeze the bottle in the middle and allow the drop to fall into the eye. Remember that it may take a few seconds between squeezing the bottle and the drop coming out. Do not apply strong pressure. If there are any doubts about administering the medicine, consult a doctor, pharmacist, or nurse.
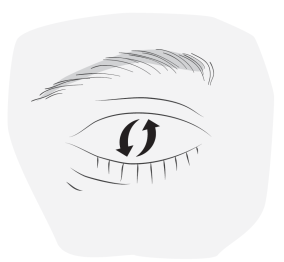
- 4. Blink several times to spread the drop throughout the eye.
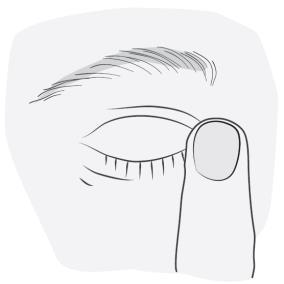
- 5. After administering the medicine, press the inner corner of the eye (on the side of the nose) with your finger for a few minutes. This helps prevent the eye drops from entering the rest of the body.
- 6. Repeat the instructions in points 2-5 to administer a drop to the other eye, if the doctor has instructed to do so. Sometimes only one eye needs treatment, and the doctor will instruct which eye needs treatment.
- 7. After use and before re-closing, shake the bottle once downwards, without touching the dropper tip, to remove any remaining liquid from the tip. This is necessary to ensure the next drops are administered correctly.
After using all the doses in the bottle, some medicine will remain. Do not worry, as the packaging contains an additional amount of Dexaprotect, and the patient will receive the full dose of Dexaprotect prescribed by the doctor. Do not try to use the excess medicine remaining in the bottle after finishing the treatment.
Frequency of use
4 to 6 times a day.
Duration of treatment
Treatment will usually involve administering drops for several days and no longer than 14 days.
Use of a higher than recommended dose of Dexaprotect
Rinse the eye with sterile water if the patient has administered too much medicine to the eye and experiences eye pain.
Immediately tell a doctor or pharmacist.
Missing a dose of Dexaprotect
Do not use a double dose to make up for a missed dose.
Stopping the use of Dexaprotect
Do not suddenly stop using this medicine. Always consult a doctor if the patient plans to stop treatment.
In case of any further doubts about using this medicine, consult a doctor, pharmacist, or nurse.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Endocrine disorders:
Unknown: frequency cannot be estimated from the available data
- increased hair growth on the body (especially in women), muscle weakness, and loss of muscle mass, purple stretch marks on the skin, increased blood pressure, irregular periods or absence of periods, changes in protein and calcium levels in the body, growth retardation in children and adolescents, and swelling and weight gain, especially on the trunk and face (a condition called Cushing's syndrome) (see section 2 "Warnings and precautions").
Eye disorders:
Very common(may affect more than 1 in 10 people):
- high pressure in the eye after 2 weeks of using the drops.
Common(may affect up to 1 in 10 people):
- discomfort, irritation, burning, stinging, itching, and blurred vision after using the medicine. These side effects are usually mild and short-term.
Uncommon(may affect up to 1 in 100 people):
- symptoms of an allergic reaction,
- prolonged healing,
- clouding of the lens (cataract),
- infections,
- high pressure in the eye (glaucoma).
Rare(may affect up to 1 in 1,000 people):
- inflammation of the surface of the eye causing redness, tearing, and irritation (conjunctivitis),
- pupil dilation,
- facial swelling,
- drooping eyelids,
- inflammation of the eye causing pain and redness (uveitis),
- calcium deposits on the surface of the eye (corneal calcification),
- inflammation of the surface of the eye causing blurred vision, dry eyes, sensitivity to light, burning, tearing, and a feeling of sand in the eye (crystalline keratopathy),
- changes in the thickness of the surface of the eye (cornea),
- swelling of the surface of the eye (corneal edema),
- ulcer on the surface of the eye causing pain, tearing, squinting, and vision loss,
- small holes in the surface of the eye (corneal perforation).
In patients with severe damage to the transparent layer on the front of the eye (cornea), phosphates may rarely cause corneal clouding due to calcium accumulation during treatment.
Reporting side effects
If side effects occur, including any side effects not listed in this leaflet, tell a doctor or pharmacist. Side effects can be reported directly to the Department of Monitoring of Adverse Reactions to Medicinal Products of the Office for Registration of Medicinal Products, Medical Devices, and Biocidal Products, Al. Jerozolimskie 181C, 02-222 Warsaw, tel.: +48 22 492 13 01, fax: +48 22 492 13 09, website: https://smz.ezdrowie.gov.pl
By reporting side effects, more information can be collected on the safety of the medicine.
Side effects can also be reported to the marketing authorization holder.
5. How to store Dexaprotect
Keep the medicine out of sight and reach of children.
Do not use the eye drops for more than 28 days after opening the bottle. Write the date of opening the bottle in the appropriate place on the label of the bottle and on the carton.
Do not use this medicine after the expiry date stated on the carton and bottle after "EXP".
The expiry date refers to the last day of the given month.
There are no special precautions for storing the medicine.
Medicines should not be disposed of via wastewater or household waste. Ask a pharmacist how to dispose of medicines that are no longer needed. This will help protect the environment.
6. Contents of the packaging and other information
What Dexaprotect contains
- The active substance is dexamethasone phosphate. One milliliter of solution contains 1 mg of dexamethasone phosphate in the form of dexamethasone sodium phosphate.
- The other ingredients are: disodium phosphate dodecahydrate, sodium chloride, disodium edetate, hydrochloric acid (to adjust pH), sodium hydroxide (to adjust pH), water for injections.
What Dexaprotect looks like and what the pack contains
Dexaprotect is a clear solution supplied in a white, opaque bottle with LDPE with a capacity of 11 ml with a Novelia system consisting of a dropper (with HDPE and silicone) and a cap with HDPE.
The following pack sizes are available: 1 or 3 bottles containing 6 ml of solution, in a cardboard box.
Not all pack sizes may be marketed.
Marketing authorization holder and manufacturer
Marketing authorization holder
S-LAB Sp. z o.o.
ul. Kiełczowska 2
55-095 Mirków
Manufacturer:
EXCELVISION
27 rue de la Lombardière
Zl La Lombardière
07100 Annonay
France
Pharmathen SA
Dervenakion 6
15351 Pallini Attiki
Greece
This medicine is authorized in the Member States of the European Economic Area under the following names:
Denmark
Oratoria
Italy
Dexavision
Cyprus
Oratoria
Greece
Oratoria
Poland
Dexaprotect
Sweden
Oratoria
Germany
Oratoria 1 mg/ml Augentropfen, Lösung
France
Dexocol
United Kingdom
Eythalm
Date of last revision of the leaflet: 09/2021
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- ImporterExcelvision Pharmathen S.A.
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to DexaprotectDosage form: Drops, 1 mg/mlActive substance: dexamethasoneManufacturer: Pharmaselect International Beteiligungs GmbHPrescription requiredDosage form: Drops, 1.315 mg/mlActive substance: dexamethasoneManufacturer: mibe GmbH ArzneimittelPrescription requiredDosage form: Drops, 1 mg/mlActive substance: dexamethasoneManufacturer: Excelevision Laboratoire UnitherPrescription required
Alternatives to Dexaprotect in other countries
The best alternatives with the same active ingredient and therapeutic effect.
Alternative to Dexaprotect in Spain
Alternative to Dexaprotect in Ukraine
Online doctors for Dexaprotect
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for Dexaprotect – subject to medical assessment and local rules.














