FRIDEX 1 mg/ml EYE DROPS IN SOLUTION IN SINGLE-DOSE CONTAINERS

How to use FRIDEX 1 mg/ml EYE DROPS IN SOLUTION IN SINGLE-DOSE CONTAINERS
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the User
Fridex 1 mg/ml eye drops, solution in single-dose container
Dexamethasone phosphate
Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the pack
- What is Fridex and what is it used for
- What you need to know before you use Fridex
- How to use Fridex
- Possible side effects
- Storage of Fridex
- Contents of the pack and further information
1. What is Fridex and what is it used for
This medicine contains the active substance dexamethasone, which is a corticosteroid indicated to stop inflammatory symptoms (pain, heat, swelling, and redness).
This medicine is indicated for the treatment of eye inflammation.
If you have an eye infection (red eye, tears, and discharge), you will be given another medicine to take at the same time as Fridex (see Section 2).
2. What you need to know before you use Fridex
Do not useFridex
- if you are allergic to dexamethasone phosphate sodium or any of the other ingredients of this medicine (listed in section 6);
- if you have an eye infection that may be caused by bacteria (acute purulent infection), fungi, or viruses (herpes virus, vaccinia virus, chickenpox virus) or caused by amoebas (a parasite);
- if you have damage to the surface of the eye (small holes, ulcers, or lesions that have not healed properly);
- if you have high pressure in the eye that is known to be caused by glucocorticoids (a family of corticosteroid medicines).
Warnings and precautions
Consult your doctor or pharmacist before starting to use this medicine.
Do not inject, do not swallow.
Avoid contact between the tip of the dispenser and the eye or eyelids.
Strict control of the eye is required during the use of Fridex, and in particular:
- for children and the elderly. More frequent monitoring is recommended.
- if you have an eye infection. Use this medicine only if you are being treated with an anti-infectious medicine at the same time.
- if you have a corneal ulcer (an open sore on the surface of the eye with sometimes extreme pain, tears, squinting, and vision loss). Do not use this medicine unless inflammation is the main cause of delayed healing.
- if you have high pressure in the eye. If you have already had high pressure in the eye after using an ocular steroid medicine, you are at risk of having it again if you use this medicine.
- if you have glaucoma, a disease that can cause damage to the optic nerve and can cause vision loss.
- children: do not use during long-term treatment without a break.
- if you have severe allergic conjunctivitis (redness, swelling, itching, and tears in the eyes) that another medicine has not been able to treat. Use this medicine only for a short period of time.
- if you have diabetes. Inform your ophthalmologist or optician about your illness before using this medicine.
- if you have a red eye that has not been diagnosed. Do not use this medicine before talking to your doctor.
- diabetic: if you are diabetic, inform your ophthalmologist or optician.
- if you have a red eye that has not been diagnosed, do not use this medicine.
- for children and the elderly. More frequent monitoring is recommended.
Avoid wearing contact lenses during the use of this medicine.
Consult your doctor if you notice swelling and weight gain around the trunk and face, as these are usually the first signs of a condition called Cushing's syndrome. A decrease in adrenal gland function may develop after stopping long-term or intensive treatment with this medicine. Consult your doctor before stopping treatment on your own. These risks are especially important in children and patients treated with a medicine called ritonavir or cobicistat.
Contact your doctor if you experience blurred vision or other visual disturbances.
Children
Do not use this medicine in children during long-term treatment without a break.
Using Fridex with other medicines
Tell your doctor or pharmacist if you are using, have recently used, or might use any other medicines.
If you are using other eye drops, wait 15 minutes before instilling the other medicine.
The combined use of eye drops containing steroids and eye drops containing beta-blockers (to treat high pressure in the eye) may cause calcium phosphate to settle on the surface of the eye.
Tell your doctor if you are using ritonavir or cobicistat, as these medicines may increase the amount of dexamethasone in the blood.
Pregnancy and breastfeeding
If you are pregnant or breastfeeding, think you may be pregnant, or are planning to have a baby, ask your doctor or pharmacist for advice before using this medicine.
There is not enough information on the use of this medicine during pregnancy to assess the possible risks. For this reason, the use of this medicine during pregnancy is not recommended.
It is not known whether this medicine passes into breast milk. However, the dose of this medicine is low, so this medicine can be used during breastfeeding.
Driving and using machines
You may have blurred vision for a short time after using this eye drop. Wait until your vision is normal before driving or using machines.
Fridex contains phosphates
This medicine contains 0.4461 mg of phosphates in 0.3 ml, which is equivalent to 1.487 mg/ml.
If you have severe damage to the clear layer on the front of the eye (the cornea), phosphates may rarely cause cloudy patches on the cornea due to calcium accumulation during treatment.
3. How to use Fridex
Follow exactly the instructions for administration of this medicine given by your doctor or pharmacist. If you are unsure, consult your doctor or pharmacist again.
Dose
The recommended dose is 1 drop, 4 to 6 times a day in the eye to be treated.
If your condition is more severe, you may be told to start with 1 drop every hour and then change to 1 drop every 4 hours, after the medicine has started to work. It is important to reduce the dose slowly to avoid the condition getting worse again.
Use in elderly patients
No dose adjustment of the medicine is required.
Use in children
Do not use this medicine in children during long-term treatment without a break.
Method of administration
This medicine must be instilled into the eye. Do not inject or swallow the medicine.
|
- Open the aluminum pouch containing 5 single-dose containers.
- Wash your hands well before using these drops.
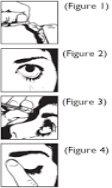
- Separate a single-dose container from the strip and open the top of the single-dose container (Figure 1).
- Tilt your head back and gently pull down the lower eyelid with your finger (Figure 2). Put one drop in the eye to be treated. Avoid contact between the tip of the container and the eye or eyelids (Figure 3).
- Immediately after putting the drop in the eye, gently press the inner corner of the eye closest to the nose with your finger for a few minutes (Figure 4). This helps prevent the eye drop from spreading to the rest of your body.
- Discard the single-dose container after use. Do not save it for later use.
Duration of treatment
You will usually need to use your drops for several days. Do not use this medicine for more than 14 days.
If you use more Fridex than you should
If you have put too much medicine in the eye and it hurts, rinse it with sterile water. Tell your doctor or pharmacist immediately.
In case of overdose or accidental ingestion, consult your doctor or pharmacist immediately or call the Toxicology Information Service, phone: 91 562 04 20, indicating the medicine and the amount ingested.
If you forget to use Fridex
Do not use a double dose to make up for forgotten doses.
If you stop using Fridex
Do not stop using this medicine suddenly. Tell your doctor if you are thinking of stopping treatment.
If you have any further questions on the use of this medicine, ask your doctor or pharmacist.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Very common(may affect more than 1 in 10 people)
- high pressure in the eye after 2 weeks of using the drops.
Common(may affect up to 1 in 10 people)
- discomfort, irritation, burning, stinging, itching, and blurred vision after use. These are usually mild and do not last long.
Uncommon(may affect up to 1 in 100 people)
- signs of an allergic reaction
- healing that takes longer than expected
- cloudy lens (cataract)
- eye infections
- high pressure in the eye (glaucoma)
- if used frequently, it is possible that the kidneys do not produce enough hormones (suppression of adrenal function). This could be shown by low blood sugar levels, dehydration, weight loss, and feeling confused about where you are.
Rare(may affect up to 1 in 10,000 people)
- inflamed surface of the eye that produces red eyes, tears, and irritation (conjunctivitis)
- dilated pupil (mydriasis)
- swelling of the face (facial edema)
- drooping eyelids (ptosis)
- inflammation of the eye that causes pain and redness (uveitis)
- calcium deposits on the surface of the eye (corneal calcification)
- inflamed surface of the eye that provides blurred vision, dry eyes, sensitivity to light, burning, tears, and a gritty feeling in the eye (crystalline keratopathy)
- changes in the thickness of the surface of the eye
- swelling of the surface of the eye (corneal edema)
- ulcer on the surface of the eye that causes pain, tears, squinting, and vision loss
- small holes in the surface of the eye (corneal perforation)
Reporting of side effects
If you experience any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. You can also report side effects directly to the Spanish Medicines Surveillance System for Human Use: www.notificaRAM.es. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of Fridex
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date which is stated on the carton. The expiry date is the last day of the month stated.
After opening the single-dose container: discard any remaining solution in the single-dose container after instillation.
Keep the single-dose containers in the pouch to protect them from light and use within 60 days.
Keep the single-dose container in the outer packaging to protect it from light.
Store below 25°C.
Medicines should not be disposed of via wastewater or household waste. Dispose of the containers and medicines you no longer need in the SIGRE collection point at the pharmacy or in any other system for collecting medicinal waste. If you are unsure, ask your pharmacist how to dispose of the containers and medicines you no longer need. This will help protect the environment.
6. Contents of the pack and further information
Composition of Fridex
- The active substance is dexamethasone phosphate sodium. Each ml of solution contains 1 mg of dexamethasone phosphate (as dexamethasone phosphate sodium), equivalent to 40 micrograms of dexamethasone phosphate (with a theoretical drop volume of approximately 40 microliters).
- The other ingredients are: disodium edetate, disodium phosphate dodecahydrate, sodium chloride, hydrochloric acid (for pH adjustment), sodium hydroxide (for pH adjustment), and water for injectable preparations.
Appearance of the product and contents of the pack
Fridex is a clear, colorless solution supplied in single-dose containers.
Each single-dose container contains 0.3 ml of solution.
A carton contains 20 or 30 single-dose containers packaged in pouches.
Marketing authorization holder and manufacturer
Marketing authorization holder
NTC S.r.l.
Via Luigi Razza, 3
20124 Milan (Italy)
Manufacturer
Farmigea SpA
Via Giovan Battista Oliva, 6/8
56121 Pisa (PI)
Italy
You can request more information about this medicine by contacting the local representative of the marketing authorization holder:
NTC Ophthalmics Iberica S.L.
Calle Pinar, 5,
28006 Madrid, Spain
This medicine is authorized in the Member States of the European Economic Area under the following names:
Portugal: Dexametasona NTC
United Kingdom: Dexamethasone phosphate 1 mg/mL (as sodium phosphate) Eye drops, Solution in a single-dose container
Spain: Fridex 1 mg/ml eye drops, solution in single-dose container
Date of last revision of this leaflet:July 2019
Detailed and up-to-date information on this medicine is available on the website of the Spanish Agency for Medicines and Health Products (AEMPS) http://www.aemps.gob.es/
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to FRIDEX 1 mg/ml EYE DROPS IN SOLUTION IN SINGLE-DOSE CONTAINERSDosage form: EYEDROP, 1 mg/mlActive substance: dexamethasoneManufacturer: Pharmaselect International Beteiligungs GmbhPrescription requiredDosage form: EYE DROP, 0.1 %Active substance: dexamethasoneManufacturer: Fidia Farmaceutici S.P.A.Prescription requiredDosage form: EYEDROP, 1mg/mlActive substance: dexamethasoneManufacturer: Laboratoires TheaPrescription required
Online doctors for FRIDEX 1 mg/ml EYE DROPS IN SOLUTION IN SINGLE-DOSE CONTAINERS
Discuss questions about FRIDEX 1 mg/ml EYE DROPS IN SOLUTION IN SINGLE-DOSE CONTAINERS, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions