
Demezon

Ask a doctor about a prescription for Demezon

How to use Demezon
LEAFLET INCLUDED IN THE PACKAGING: INFORMATION FOR THE USER
Demezon, 1.315 mg/ml, eye drops in a single-dose container
Dexamethasone sodium phosphate
You should carefully read the contents of the leaflet before using the medicine, as it contains important information for the patient.
- You should keep this leaflet, so that you can read it again if you need to.
- In case of any doubts, you should consult a doctor or pharmacist.
- This medicine has been prescribed specifically for you. Do not pass it on to others. The medicine may harm another person, even if their symptoms are the same.
- If the patient experiences any side effects, including those not listed in this leaflet, they should tell their doctor or pharmacist. See section 4.
Table of contents of the leaflet
- 1. What is Demezon and what is it used for
- 2. Important information before using Demezon
- 3. How to use Demezon
- 4. Possible side effects
- 5. How to store Demezon
- 6. Contents of the pack and other information
1. What is Demezon and what is it used for
Demezon contains a corticosteroid that soothes inflammatory reactions in the eye, resulting from e.g. an allergy.
Demezon is used to treat non-infectious inflammatory conditions of the conjunctiva, cornea, and anterior segment of the eye, including allergies, irritations, thermal and chemical burns.
2. Important information before using Demezon
When not to use Demezon:
- if the patient is allergic to dexamethasone sodium phosphate or any of the other ingredients of this medicine (listed in section 6),
- if the patient has injuries or corneal ulcers
- if the patient has increased intraocular pressure (glaucoma)
- if the patient has viral infections (herpetic keratitis, chickenpox, shingles or other viral eye infections), untreated bacterial infections, fungal infections (Candida albicans), or untreated parasitic eye infections.
- if the patient has tuberculous eye infection.
In case of infection, the use of Demezon is recommended only with concurrent anti-infective treatment.
Warnings and precautions
Before starting to use Demezon, you should discuss it with your doctor or pharmacist.
For use in the eyes only.
During prolonged use of Demezon:
- Intraocular pressure (in the eyes) may be increased. If you are using Demezon, your intraocular pressure should be monitored periodically. You should ask your doctor for advice. This is especially important in pediatric patients, as the risk of corticosteroid-induced ocular hypertension is higher in children and may occur earlier than in adults. In predisposed patients (e.g. those with diabetes), the risk of corticosteroid-induced increased intraocular pressure and/or cataract is increased.
- Cataract (lens clouding) may develop
- Cushing's syndrome may develop, as Demezon may be absorbed into the bloodstream. You should consult your doctor if you experience swelling and weight gain, particularly on the trunk and face, as these are usually the first symptoms of Cushing's syndrome. Adrenal insufficiency may occur as a result of discontinuation of prolonged or intensive use of Demezon. You should consult your doctor before deciding to discontinue treatment. This risk is particularly important in children and patients treated with ritonavir or cobicistat.
In case of prolonged use of the product, you should regularly consult your doctor.
In case of worsening or sudden onset of symptoms, you should contact your doctor immediately. Patients using Demezon may be more susceptible to eye infections.
If an infection occurs, your doctor will prescribe appropriate anti-infective drugs.
Corticosteroids used in the eyes may delay wound healing in the eye. Topically applied non-steroidal anti-inflammatory drugs (NSAIDs) may also slow down or delay wound healing. Concurrent administration of topically applied NSAIDs and topically applied corticosteroids increases the risk of wound healing disorders.
If you have diseases that lead to thinning of the eye tissues, you should consult your doctor or pharmacist before using Demezon..
If you experience blurred vision or other vision disturbances, you should contact your doctor.
Demezon and other medicines
You should tell your doctor or pharmacist about all medicines you are currently using or have recently used, as well as any medicines you plan to use.
You should inform your doctor or pharmacist about the use of topically applied NSAIDs. Concurrent use of topically applied corticosteroids and topically applied NSAIDs may increase wound healing problems.
You should tell your doctor about taking ritonavir or cobicistat, as these drugs may increase the levels of dexamethasone in the blood.
When using Demezon and other eye drops in the treatment of inflammatory conditions of the uvea and sclera (atropine and other anticholinergic drugs), it is not possible to exclude an additional increase in intraocular pressure in appropriately predisposed patients.
Pregnancy and breastfeeding
If you are pregnant or breastfeeding, think you may be pregnant, or plan to have a child, you should consult your doctor or pharmacist before using this medicine.
Pregnancy
There is insufficient evidence on the safety of using Demezon during pregnancy. Therefore, it is not recommended to use Demezon during pregnancy.
Breastfeeding
It is not known whether the use of Demezon leads to significant absorption into the body and, consequently, into breast milk. Therefore, it is not recommended to use Demezon during breastfeeding. When using higher doses or in case of prolonged treatment, breastfeeding should be discontinued.
Driving and using machines
Demezon has no influence or negligible influence on the ability to drive and use machines. During use of Demezon, temporary blurred vision may occur after eye drop administration. Patients should not drive, work without proper protection, or operate machines until these disturbances have resolved.
Warning for contact lens wearers.
Wearing contact lenses is not recommended during the treatment of eye inflammatory conditions, as the inflammation may worsen. If your doctor allows you to wear contact lenses, you should remove them before application and wait at least 15 minutes after application before reinserting them.
Demezon contains phosphates
The medicine contains 4.36 mg of phosphates in each 1 ml of eye drops.
In patients with severe damage to the transparent, anterior part of the eye (cornea), phosphates may, in very rare cases, cause corneal clouding due to calcium deposition during treatment.
3. How to use Demezon
This medicine should always be used exactly as your doctor or pharmacist has told you. If you are not sure, you should ask your doctor or pharmacist.
Recommended dose
Unless your doctor has prescribed otherwise, the recommended dose is:
For the first 2 days, use 1 drop 2 to 5 times a day into the conjunctival sac, and then 1 drop 3 times a day. In severe cases, initially 1 drop every hour.
The duration of treatment will be determined by your doctor, depending on the severity of the condition and the progression of the disease. However, the duration of treatment should not exceed 2 weeks without consulting your doctor.
Method of administration
For use in the eyes only.
Wash your hands.
Separate the single-dose container from the others by gently twisting and pulling.
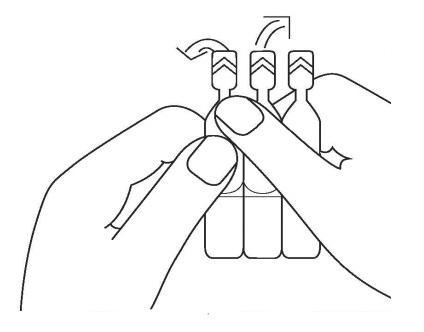
Twist the tip. Only by twisting (and not pulling) will an opening be created to form the dropper.
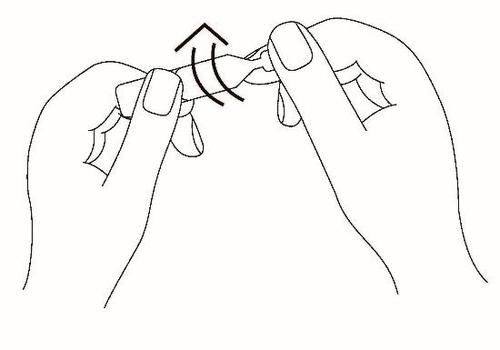
Tilt your head back slightly, look up, and with your free hand, pull the lower eyelid down. Hold the single-dose container vertically over the eye. Gently squeeze the container to administer the medicine. Do not touch the dropper tip to the eye or eyelid.
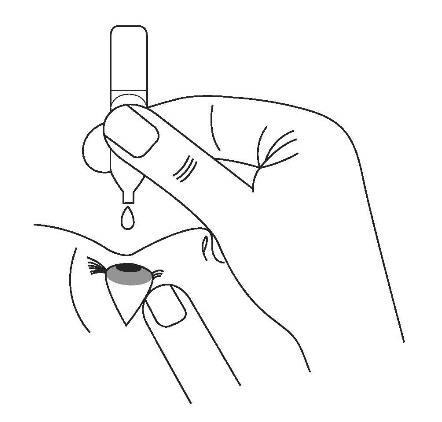
The following procedure will help minimize the amount of medicine that gets into the bloodstream after using the eye drops: close your eyes and at the same time use your finger to block the tear duct, gently pressing for at least one minute.
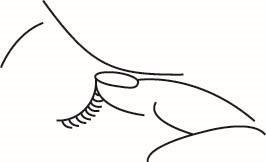
If the drop did not get into the eye, you should administer the next drop.
Using a higher dose of Demezon than recommended
Flush your eyes with lukewarm water. Do not use the drops until the next scheduled dose.
Due to the limited capacity of the conjunctival sac, it is practically impossible to introduce too much Demezon into the eye.
Missing a dose of Demezon
You should continue treatment according to the established dosing schedule. Do not use a double dose to make up for a missed dose.
If you are using other eye drops or ointments in addition to Demezon, you should wait at least 5 minutes between administrations of the next medicines. Eye ointments should be used last.
If you have any further doubts about using this medicine, you should consult your doctor or pharmacist.
4. Possible side effects
Like all medicines, Demezon can cause side effects, although not everybody gets them.
In clinical trials, the most common adverse reaction was eye discomfort.
The following side effects have been observed during the use of Demezon:
- eye discomfort
Uncommon (may affect up to 1 in 100 people)
- taste disturbances
- inflammation of the eye surface, dry eye, sensitivity to light, blurred vision, unusual sensations in the eyes, increased tearing, crusts on the eyelid margins, eye itching, irritation, or redness of the eye
Frequency not known (frequency cannot be estimated from the available data)
- allergic reactions
- adrenal insufficiency, hormonal disturbances: excessive hair growth on the body (especially in women), muscle weakness, and muscle mass loss, purple striae on the skin, increased blood pressure, irregular menstrual periods or amenorrhea, changes in protein and calcium levels in the body, growth retardation in children and adolescents, and swelling and weight gain, particularly on the trunk and face (Cushing's syndrome) (see section 2 "Warnings and precautions")
- dizziness, headache
- glaucoma, corneal ulcers, increased intraocular pressure, decreased visual acuity, corneal damage, eyelid ptosis, eye pain, pupil dilation, permanent cataract formation
Description of selected side effects
Prolonged use of corticosteroids administered locally to the eyes may cause increased intraocular pressure with damage to the optic nerve head, decreased visual acuity, visual field disturbances, and partial cataract formation.
Due to the corticosteroid content, in cases of conditions involving corneal or scleral thinning, there is a higher risk of perforation, especially after prolonged treatment.
Corticosteroids may decrease resistance to infections and promote their occurrence.
In patients with significantly damaged anterior, transparent part of the eye (cornea), very rare cases of corneal clouding have been observed due to calcium deposition during treatment.
Reporting side effects
If you experience any side effects, including those not listed in the leaflet, you should tell your doctor or pharmacist. Side effects can be reported directly to the Department of Drug Safety Monitoring of the Office for Registration of Medicinal Products, Medical Devices, and Biocidal Products
Jerozolimskie Avenue 181C
02-222 Warsaw
Phone: +48 22 49 21 301
Fax: +48 22 49 21 309
Website: https://smz.ezdrowie.gov.pl
Side effects can also be reported to the marketing authorization holder.
By reporting side effects, you can help provide more information on the safety of this medicine.
5. How to store Demezon
The medicine should be stored out of sight and reach of children.
Store in the original packaging to protect from light.
After opening the protective foil sachet, do not use the eye drops after 6 months.
Do not use this medicine after the expiry date stated on the single-dose container, foil sachet, and cardboard box after: EXP. The expiry date stated is the last day of the given month.
The eye drops should be used immediately after opening. Single-dose containers are intended for single use only. Any remaining solution after single use should be discarded.
Medicines should not be disposed of via wastewater or household waste. You should ask your pharmacist how to dispose of medicines that are no longer needed. This will help protect the environment.
6. Contents of the pack and other information
What Demezon contains
- The active substance of the medicine is dexamethasone sodium phosphate. 1 ml of solution contains 1.315 mg of dexamethasone sodium phosphate, equivalent to 1 mg of dexamethasone (1.2 mg of dexamethasone phosphate).
- The other ingredients are: sodium chloride, anhydrous disodium phosphate, disodium dihydrogen phosphate dihydrate, disodium edetate, water for injections.
What Demezon looks like and contents of the pack
Demezon is a clear, colorless to slightly yellowish solution.
The single-dose container made of LDPE is placed in a sachet made of PETP/Aluminum/LDPE. The sachet contains 5 single-dose containers. The whole is in a cardboard box.
Package sizes: 10 or 20 single-dose plastic containers of 0.4 ml of eye drop solution.
Not all package sizes may be marketed.
Marketing authorization holder and manufacturer
Marketing authorization holder
SUN-FARM Sp. z o.o.
Dolna 21
05-092 Łomianki
Manufacturer
mibe GmbH Arzneimittel
Münchener Straße 15
06796 Brehna
Germany
This medicinal product is authorized in the Member States of the European Economic Area under the following names:
Countries of the European Economic Area
Germany – Dexafluid® sine
Poland – Demezon
Date of last revision of the leaflet:12.2020
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- Importermibe GmbH Arzneimittel
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to DemezonDosage form: Drops, 1 mg/mlActive substance: dexamethasoneManufacturer: Pharmaselect International Beteiligungs GmbHPrescription requiredDosage form: Drops, 1 mg/mlActive substance: dexamethasoneManufacturer: Excelevision Laboratoire UnitherPrescription requiredDosage form: Drops, 1 mg/mlActive substance: dexamethasonePrescription required
Alternatives to Demezon in other countries
The best alternatives with the same active ingredient and therapeutic effect.
Alternative to Demezon in Spain
Alternative to Demezon in Ukraine
Online doctors for Demezon
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for Demezon – subject to medical assessment and local rules.














