
Catalin
Ask a doctor about a prescription for Catalin

How to use Catalin
Leaflet attached to the packaging: information for the user
Catalin
0.75 mg, tablet and solvent for preparing eye drops
Pirenoxine
Please read the contents of the leaflet carefully before using the medicine, as it contains important information for the patient.
- Please keep this leaflet, so that you can read it again if necessary.
- In case of any doubts, please consult a doctor or pharmacist.
- This medicine has been prescribed specifically for this person. Do not pass it on to others. The medicine may harm another person, even if their symptoms are the same.
- If the patient experiences any side effects, including any side effects not listed in this leaflet, they should inform their doctor or pharmacist. See section 4.
Table of contents of the leaflet:
- 1. What is Catalin and what is it used for
- 2. Important information before using Catalin
- 3. How to use Catalin
- 4. Possible side effects
- 5. How to store Catalin
- 6. Contents of the packaging and other information
1. What is Catalin and what is it used for
Catalin is indicated for the treatment of age-related cataracts. The active substance of Catalin is pirenoxine. The packaging contains a tablet and a solvent; the tablet is light orange and the solvent is clear and colorless. Although the cause of cataract formation has not been fully explained, it is believed that lens clouding occurs due to the conversion of its soluble proteins into insoluble proteins. Catalin inhibits and prevents the progression of cataracts.
2. Important information before using Catalin
When not to use Catalin
If the patient is allergic to pirenoxine or any of the other ingredients of this medicine (listed in section 6).
Warnings and precautions
Before starting to use Catalin, the patient should discuss it with their doctor or pharmacist.
Catalin and other medicines
The patient should tell their doctor about all medicines they are currently taking or have recently taken, as well as any medicines they plan to take. There is no data on interactions with other medicines or other types of interactions.
Using Catalin with food, drink, and alcohol
Not applicable.
Pregnancy, breastfeeding, and fertility
If the patient is pregnant or breastfeeding, thinks they may be pregnant, or plans to have a child, they should consult their doctor before using this medicine. There is no data on the safety of using Catalin during pregnancy and breastfeeding.
Driving and using machines
Catalin does not affect the ability to drive or use machines.
Catalin contains excipients: methyl parahydroxybenzoate, propyl parahydroxybenzoate
which may cause allergic reactions (possible late reactions).
3. How to use Catalin
This medicine should always be used according to the doctor's or pharmacist's recommendations. In case of doubts, the patient should consult their doctor or pharmacist.
Method of preparing the solution
Dissolve the tablet in 15 ml of solvent. A clear, yellow solution is formed.
Available packaging1 tablet and 15 ml of solvent.
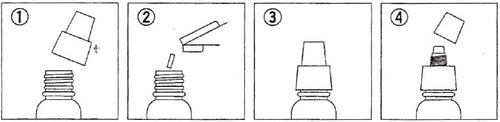
Remove the white
cap from the
bottle.
Open the
tablet packaging and
put it into the
bottle. Do not
touch the tablet
directly.
Before use,
remove the brown
stopper.
Instill into the eye.
Do not touch the eye
with the dropper tip.
Close the bottle
tightly and
shake until the
tablet is dissolved.
Method of administration:
- 1. Prepare the bottle with the medicine and a mirror.
- 2. Wash your hands.
- 3. Remove the stopper.
- 4. Take the bottle in your hand and point it upwards, holding it with your thumb and middle finger.
- 5. Tilt your head back. With a clean finger, pull the lower eyelid down to form a "pocket"; the drop should fall into it.
- 6. Bring the dropper tip close to the eye. You can use a mirror to help.
- 7. Do not touch the dropper tip to the eye, eyelid, or surrounding areas.This can cause infection. Using infected drops can lead to serious complications, even vision loss.
- 8. To release a single drop of the medicine, gently squeeze the bottom of the bottle.
- 9. If the drop does not fall into the eye, try again.
- 10. After instilling the medicine, gently close your eye and press the corner of your eye near your nose for at least one minute. This will help prevent the medicine from entering the entire body.
- 11. If using drops for both eyes, repeat the above procedure to instill the drop into the other eye.
- 12. Immediately after using the medicine, put the stopper back on.
Dosage
Instill 1 or 2 drops into the conjunctival sac 3 to 5 times a day.
Using a higher dose of Catalin than recommended
There are no known symptoms of overdose of Catalin in humans. An antidote is not necessary, as overdosing on pirenoxine is practically impossible.
4. Possible side effects
Like all medicines, Catalin can cause side effects, although not everybody gets them.
If the patient experiences any allergic reactions, including rash, swelling of the face, lips, tongue, and/or throat, which may cause difficulty breathing or swallowing, or other serious side effects, they should stop using Catalin and contact their doctor or the Emergency Department of the nearest hospital immediately.
During treatment with Catalin, the following may occur:
Rarely (less than 1 in 1000 people):
diffuse superficial keratitis, blepharitis, conjunctival hyperemia, pain, itching.
Reporting side effects
If any side effects occur, including any side effects not listed in this leaflet, the patient should inform their doctor, pharmacist, or nurse. Side effects can be reported directly to the Department of Monitoring of Adverse Reactions to Medicinal Products of the Office for Registration of Medicinal Products, Medical Devices, and Biocidal Products:
Al. Jerozolimskie 181C,
02-222 Warsaw,
tel.: +48 22 49 21 301
fax: +48 22 49 21 309
e-mail: [email protected]
Side effects can also be reported to the marketing authorization holder.
By reporting side effects, more information can be gathered on the safety of the medicine.
5. How to store Catalin
The medicine should be stored out of sight and reach of children.
Do not use this medicine after the expiry date stated on the carton and label.
Store in a temperature below 25°C. After preparing the solution, store it in a cool place, protected from light. Shelf life after dissolving the tablet: 20 days.
6. Contents of the packaging and other information
What Catalin contains
The active substance of Catalin is pirenoxine in the form of sodium salt, in a dose of 0.75 mg.
The other ingredients are:
- in the tablet: taurine, boric acid;
- in the solvent: boric acid, potassium chloride, sodium tetraborate, methyl parahydroxybenzoate, propyl parahydroxybenzoate, purified water.
What Catalin looks like and what the packaging contains
The carton contains a tablet placed in a blister pack made of Al/PCV film and a solvent placed in a 15 ml PP bottle. The tablet is light orange, and the solvent is clear and colorless.
Marketing authorization holder
Senju Poland Sp. z o.o.
Rondo Ignacego Daszyńskiego 2B,
00-843 Warsaw
tel.: +48 22 236 05 05
e-mail: [email protected]
Importer who releases the series:
PROFARM PS Sp. z o.o.
ul. Słoneczna 96
05-500 Stara Iwiczna
FARMACOL S.A.
ul. Szopienicka 77
40-431 Katowice
Synoptis Industrial Sp. z o.o.
ul. Rabowicka 15
62-020 Swarzędz
To obtain more detailed information, please contact the marketing authorization holder.
Date of last update of the leaflet:07/2024
- Country of registration
- Prescription requiredYes
- ImporterFARMACOL S.A. PROFARM PS Sp. z o.o. Synoptis Industrial Sp. z o.o.
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to CatalinDosage form: Drops, 3.2 mg/mlActive substance: artificial tears and other indifferent preparationsPrescription not requiredDosage form: Gel, 50 mg/gActive substance: dexpanthenolPrescription requiredDosage form: Gel, 50 mg/gActive substance: dexpanthenolPrescription required
Alternatives to Catalin in other countries
The best alternatives with the same active ingredient and therapeutic effect.
Alternative to Catalin in Ukraine
Alternative to Catalin in Spain
Online doctors for Catalin
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for Catalin – subject to medical assessment and local rules.














