
LUXTURNA 5 x 10¹² GENE VECTOR UNITS/ML CONCENTRATE AND SOLVENT FOR INJECTABLE SOLUTION

How to use LUXTURNA 5 x 10¹² GENE VECTOR UNITS/ML CONCENTRATE AND SOLVENT FOR INJECTABLE SOLUTION
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the Patient
Luxturna 5× 1012vector genomes/ml concentrate and solvent for solution for injection
voretigene neparvovec
This medicinal product is subject to additional monitoring, which will allow for quick identification of new safety information. You can help by reporting any side effects you may get. See the end of section 4 for how to report side effects.
Read all of this leaflet carefully before you are given this medicine because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor or nurse.
- If you get any side effects, talk to your doctor or nurse. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the pack:
- What Luxturna is and what it is used for
- What you need to know before you are given Luxturna
- How Luxturna is given
- Possible side effects
- How to store Luxturna
- Contents of the pack and other information
1. What Luxturna is and what it is used for
Luxturna is a gene therapy product that contains the active substance voretigene neparvovec.
Luxturna is used to treat adults and children with vision loss due to inherited retinal dystrophy caused by mutations in the RPE65gene. These mutations prevent the body from producing a protein necessary for vision, leading to vision loss and possible blindness.
The active substance in Luxturna, voretigene neparvovec, is a modified virus that contains a copy of the RPE65gene. After injection, this gene reaches the cells of the retina, the layer at the back of the eye that detects light. This allows the retina to produce the necessary proteins for vision. The virus used to deliver the gene does not cause disease in humans.
You will only be given Luxturna if genetic tests show that your vision loss is caused by mutations in the RPE65gene.
2. What you need to know before you are given Luxturna
You must not be given Luxturna
- If you are allergic to voretigene neparvovec or any of the other ingredients of this medicine (listed in section 6)
- If you have an eye infection
- If you have eye inflammation
If you are affected by any of these conditions, or if you are not sure, consult your doctor before you are given Luxturna.
Warnings and precautions
Before you receive treatment with Luxturna:
- Tell your doctor if you have signs of eye infection or inflammation, such as eye redness, sensitivity to light, eye swelling, or eye pain.
- Tell your doctor if you have an active infection of any kind. Your doctor may delay your treatment until the infection has cleared, as this medicine may make it harder for your body to fight the infection. See also section 3.
After you receive treatment with Luxturna:
- Consult your doctor immediately if your eye/eyes become red, if you feel eye pain, sensitivity to light, see flashes or floaters, or if you notice worsening or blurred vision.
- You should avoid air travel or other travel to high altitudes until your doctor tells you it is safe to do so. During treatment with this medicine, your doctor will insert an air bubble into your eye, which your body will slowly absorb. Until the air bubble has been completely absorbed, air travel or other travel to high altitudes may cause the air bubble to expand and cause damage to your eyes, including vision loss. Consult your doctor before traveling.
- You should avoid swimming due to an increased risk of eye infection. Consult your doctor before swimming after receiving treatment with Luxturna.
- You should avoid strenuous physical activity due to an increased risk of eye injury. Consult your doctor before starting strenuous physical activity after receiving treatment with Luxturna.
- You may experience temporary vision changes, such as sensitivity to light and blurred vision. Tell your doctor about any vision changes you experience. Your doctor may be able to help you reduce any discomfort caused by these temporary changes.
- The active substance in Luxturna may be temporarily excreted in your tears. You and your caregiver should place dressings and waste material that have come into contact with tears and nasal secretions in sealed bags before disposing of them. You should follow these precautions for 14 days.
- You will not be able to donate blood, organs, tissues, and cells for transplantation after being treated with Luxturna.
Children and adolescents
Luxturna has not been studied in children under 4 years of age. The data are limited.
Other medicines and Luxturna
Tell your doctor if you are taking, have recently taken, or might take any other medicines.
Pregnancy, breast-feeding, and fertility
If you are pregnant or breast-feeding, think you may be pregnant, or are planning to have a baby, ask your doctor or nurse for advice before receiving treatment with Luxturna.
The effects of this medicine on pregnancy and the fetus are not known. As a precaution, you should not receive Luxturna while you are pregnant.
Luxturna has not been studied in breast-feeding women. It is not known whether Luxturna passes into breast milk. If you are breast-feeding or plan to breast-feed, tell your doctor. Your doctor will help you decide whether to stop breast-feeding or not receive Luxturna, taking into account the benefits of breast-feeding for your baby and the benefits of Luxturna for you.
Driving and using machines
You may experience temporary vision changes after receiving Luxturna. Do not drive or use heavy machinery until your vision has recovered. Consult your doctor before resuming these activities.
Luxturna contains sodium
This medicine contains less than 1 mmol of sodium (23 mg) per dose, which is essentially "sodium-free".
3. How Luxturna is given
Luxturna will be given to you in an operating room by surgeons with experience in performing eye surgery. Luxturna is given under anesthesia. Your doctor will talk to you about the anesthesia and how it will be given.
Your doctor will perform eye surgery to remove the clear gel that fills the inside of your eye and then inject Luxturna directly into your retina, the thin layer of light-sensitive tissue at the back of your eye. This procedure will be repeated in the other eye at least 6 days later. You will need to stay in the post-operative observation area for a few hours after each procedure to monitor your recovery and watch for side effects of the surgery or anesthesia.
Before starting treatment with Luxturna, your doctor may ask you to take a medicine that suppresses your immune system (your body's natural defenses) so that it does not try to fight Luxturna when it is given. It is important that you take this medicine as directed by your doctor. Do not stop taking the medicine without first talking to your doctor.
If you are given more Luxturna than you should
As this medicine is given to you by a doctor, it is unlikely that you will be given too much medicine. If this happens, your doctor will treat any symptoms as needed. Tell your doctor or nurse if you have any vision problems.
If you have any further questions on the use of this medicine, ask your doctor or nurse.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
The following side effects may occur with Luxturna:
Common (may affect up to 1 in 10 people)
- Deposits under the retina
Frequency not known (cannot be estimated from the available data)
- Atrophy of the (chorio)retina
The following side effects may occur with the injection procedure:
Very common (may affect more than 1 in 10 people)
- Eye redness
- Cataract (clouding of the lens)
- Increased pressure in the eye
Common (may affect up to 1 in 10 people)
- Retinal tear
- Eye pain
- Eye swelling
- Retinal detachment
- Bleeding in the back of the eye
- Pain or increased discomfort in the eye
- Blurred vision due to a hole in the retina
- Thinning of the surface of the eye (dellen)
- Eye irritation
- Eye inflammation
- Feeling of something in the eye
- Eye discomfort
- Abnormalities in the back of the eye
- Nausea (feeling sick), vomiting, abdominal (stomach) pain, lip pain
- Changes in heart electrical activity
- Headache, dizziness
- Rash, facial swelling
- Anxiety
- Problems associated with the placement of a breathing tube in the trachea
- Surgical wound rupture
Not known (frequency cannot be estimated from the available data)
- Clouding of the gel-like substance inside the eye (vitreous opacities)
- Atrophy of the (chorio)retina
Damage to the tissues of the eye may be accompanied by bleeding, inflammation, and an increased risk of infection. There is a reduction in vision in the days following surgery that usually improves; tell your doctor if your vision does not return.
Reporting of side effects
If you experience any side effects, talk to your doctor or nurse, even if they are not listed in this leaflet. You can also report side effects directly via the national reporting system listed in Appendix V. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of Luxturna
Luxturna will be stored by healthcare professionals at their healthcare facility.
The concentrate and solvent should be transported and stored frozen at a temperature ≤ -65 °C. Once thawed, the medicine should not be re-frozen and should be kept at room temperature (below 25 °C).
Do not use this medicine after the expiry date which is stated on the label and carton after EXP.
6. Container Contents and Additional Information
Luxturna Composition
- The active substance is voretigene neparvovec. Each ml of concentrate contains 5 × 10^12 vector genomes (vg). The concentrate (single-dose vial of 2 ml with an extractable volume of 0.5 ml) requires a 1:10 dilution before administration.
- Each dose of diluted solution contains 1.5 × 10^11 vector genomes of voretigene neparvovec in an administrable volume of 0.3 ml.
- The other excipients of the concentrate are sodium chloride (see "Luxturna contains sodium" at the end of section 2 of this prospectus), sodium dihydrogen phosphate monohydrate (to adjust pH), disodium phosphate dihydrate (to adjust pH), poloxamer 188, and water for injectable preparations.
- The solvent contains sodium chloride (see the end of section 2), sodium dihydrogen phosphate monohydrate (to adjust pH), disodium phosphate dihydrate (to adjust pH), poloxamer 188, and water for injectable preparations.
This medicinal product contains genetically modified organisms.
Appearance of Luxturna and Container Contents
Luxturna is a clear and colorless concentrate for solution for subretinal injection, presented in a transparent plastic vial. The solvent is a clear and colorless liquid presented in a transparent plastic vial.
Each aluminum pouch contains a cardboard box that includes 1 vial of 0.5 ml of concentrate and 2 vials of solvent (each containing 1.7 ml).
Marketing Authorization Holder
Novartis Europharm Limited
Vista Building
Elm Park, Merrion Road
Dublin 4
Ireland
Manufacturer
Novartis Pharma GmbH
Roonstrasse 25
90429 Nuremberg
Germany
Novartis Pharma GmbH
Sophie-Germain-Strasse 10
90443 Nürnberg
Germany
For further information on this medicinal product, please contact the local representative of the marketing authorization holder:
Belgium/Belgique/Belgien Novartis Pharma N.V. Tel: +32 2 246 16 11 | Lithuania UAB Novartis Baltics Lietuvos filialas Tel: +370 5 269 16 50 |
| Luxembourg/Luxemburg Novartis Pharma N.V. Tel: +32 2 246 16 11 |
Czech Republic Novartis s.r.o. Tel: +420 225 775 111 | Hungary Novartis Hungária Kft. Tel: +36 1 457 65 00 |
Denmark Novartis Healthcare A/S Tel: +45 39 16 84 00 | Malta Novartis Pharma Services Inc. Tel: +356 2122 2872 |
Germany Novartis Pharma GmbH Tel: +49 911 273 0 | Netherlands Novartis Pharma B.V. Tel: +31 88 04 52 111 |
Estonia Novartis Baltics Eesti filiaal Tel: +372 66 30 810 | Norway Novartis Norge AS Tel: +47 23 05 20 00 |
Greece Novartis (Hellas) A.E.B.E. Tel: +30 210 281 17 12 | Austria Novartis Pharma GmbH Tel: +43 1 86 6570 |
Spain Novartis Farmacéutica, S.A. Tel: +34 93 306 42 00 | Poland Novartis Poland Sp. z o.o. Tel: +48 22 375 4888 |
France Novartis Pharma S.A.S. Tel: +33 1 55 47 66 00 | Portugal Novartis Farma - Produtos Farmacêuticos, S.A. Tel: +351 21 000 8600 |
Croatia Novartis Hrvatska d.o.o. Tel: +385 1 6274 220 | Romania Novartis Pharma Services Romania SRL Tel: +40 21 31299 01 |
Ireland Novartis Ireland Limited Tel: +353 1 260 12 55 | Slovenia Novartis Pharma Services Inc. Tel: +386 1 300 75 50 |
Iceland Vistor hf. Tel: +354 535 7000 | Slovakia Novartis Slovakia s.r.o. Tel: +421 2 5542 5439 |
Italy Novartis Farma S.p.A. Tel: +39 02 96 54 1 | Finland Novartis Finland Oy Tel: +358 (0)10 6133 200 |
Cyprus Novartis Pharma Services Inc. Tel: +357 22 690 690 | Sweden Novartis Sverige AB Tel: +46 8 732 32 00 |
Latvia Novartis Baltics Tel: +371 67 887 070 |
Date of Last Revision of this Prospectus:
Other Sources of Information
This prospectus is available in audio file format and in large print on the website: http://www.voretigeneneparvovec.support
Detailed information on this medicinal product is available on the European Medicines Agency website: http://www.ema.europa.eu.
------------------------------------------------------------------------------------------------------------------------
This information is intended for healthcare professionals only:
Precautions to be taken before handling or administering the medicinal product
This medicinal product contains genetically modified organisms. Personal protective equipment (including laboratory coat, safety glasses, and gloves) should be worn when preparing or administering voretigene neparvovec.
Intraocular pressure should be adequately controlled and monitored before and after administration of the medicinal product.
After administration, patients should be instructed to immediately report any symptoms suggesting endophthalmitis or retinal detachment and should be treated accordingly.
Pre-administration preparation
Each package containing 1 vial of concentrate and 2 vials of solvent is for single use only.
Luxturna should be inspected visually before administration. If particles, turbidity, or discoloration are detected, the single-dose vial should not be used.
Preparation of Luxturna should be performed within 4 hours prior to the start of the administration procedure, under aseptic conditions, and according to the following recommended procedure.
Allow one single-dose vial of concentrate and two vials of solvent to thaw at room temperature. Once the 3 vials (1 vial of concentrate and 2 vials of solvent) have thawed, dilution should be initiated. Gently invert the vials five times to mix the contents.
Inspect for visible particles or any anomaly. The appearance of any anomaly or visible particles should be reported to the Marketing Authorization Holder, and the product should not be used.
Transfer 2.7 ml of solvent from the two thawed vials and dispense with a 3 ml syringe into a sterile 10 ml glass vial.
For dilution, withdraw 0.3 ml of thawed concentrate with a 1 ml syringe and add to the 10 ml sterile vial containing the solvent. Gently invert the vial at least five times for adequate mixing. Inspect for visible particles. The diluted solution should be clear or slightly opalescent. Label the 10 ml glass vial containing the diluted concentrate as "Luxturna diluted".
Syringes should not be prepared if the vial shows any damage or if visible particles are observed. Prepare syringes for injection by withdrawing 0.8 ml of the diluted solution into a sterile 1 ml syringe. Repeat the same procedure to prepare a backup syringe. The filled syringes should be transported to the operating room in a designated container for this purpose.
Measures to be taken in case of accidental exposure
Accidental exposure should be avoided. Local biosafety regulations for the preparation, administration, and handling of voretigene neparvovec should be followed.
- Personal protective equipment (including laboratory coat, safety glasses, and gloves) should be worn when handling or administering voretigene neparvovec.
- Accidental exposure to voretigene neparvovec, including skin contact, eye contact, and mucous membrane contact, should be avoided. Any wounds should be covered before handling this medicinal product.
- Any spillage of voretigene neparvovec should be treated with a virucidal agent, such as 1% sodium hypochlorite, and dried with absorbent materials.
- All materials that may have come into contact with voretigene neparvovec (e.g., vial, syringe, needle, cotton balls, gloves, masks, or dressings) should be disposed of according to local biosafety regulations.
Accidental exposure
- In case of accidental occupational exposure (e.g., splashes in the eyes or mucous membranes), rinse with clean water for at least 5 minutes.
- In case of exposure to broken skin or needlestick injury, clean the affected area thoroughly with water and soap and/or a disinfectant.
Precautions to be taken for the disposal of the medicinal product
This medicinal product contains genetically modified organisms. Disposal of unused medicinal product and all materials that have come into contact with it should be performed according to local regulations for pharmaceutical waste.
Dosage
Treatment should be initiated and administered by a retina surgeon with experience in macular surgery.
Patients will receive a single dose of voretigene neparvovec of 1.5 × 10^11 vector genomes in each eye. Each dose will be administered within the subretinal space in a total volume of 0.3 ml. Administration should be performed individually in each eye on separate days, with an interval of at least 6 days between each surgical procedure.
Immunomodulatory regimen
Before initiating the immunomodulatory regimen and before administration of voretigene neparvovec, the patient should be examined for symptoms of active infectious disease of any nature, and if such infection is present, treatment should be postponed until after the patient has recovered.
It is recommended to initiate the immunomodulatory regimen 3 days before administration of voretigene neparvovec in the first eye, following the schedule described below (Table 1). The initiation of the immunomodulatory regimen for the second eye should follow the same scheme and should replace the immunomodulatory regimen for the first eye.
Table 1: Pre- and post-operative immunomodulatory regimen for each eye
Preoperative | 3 days before administration of Luxturna | Prednisone (or equivalent) 1 mg/kg/day (up to a maximum of 40 mg/day) |
Postoperative | 4 days (including the day of administration) | Prednisone (or equivalent) 1 mg/kg/day (up to a maximum of 40 mg/day) |
Continue for 5 days | Prednisone (or equivalent) 0.5 mg/kg/day (up to a maximum of 20 mg/day) | |
Continue for 5 days with a dose every other day | Prednisone (or equivalent) 0.5 mg/kg every other day (up to a maximum of 20 mg/day) |
Special populations
Elderly patients
The safety and efficacy of voretigene neparvovec have not been established in patients ≥ 65 years. Data are limited. However, no dose adjustment is necessary in elderly patients.
Hepatic and renal impairment
The safety and efficacy of voretigene neparvovec have not been established in patients with hepatic or renal impairment. No dose adjustment is necessary in these patients (see section 5.2).
Pediatric population
The safety and efficacy of voretigene neparvovec have not been established in children under 4 years of age. Data are limited. No dose adjustment is necessary in pediatric patients.
Method of administration
Subretinal use.
Luxturna is a sterile concentrated solution for subretinal injection that requires thawing and dilution before administration.
This medicinal product should not be administered via intravitreal injection.
Luxturna is a single-use vial for single administration in one eye. The product is administered via a subretinal injection after performing a vitrectomy in each eye. It should not be administered too close to the fovea to maintain foveal integrity.
Administration of voretigene neparvovec should be performed in the operating room under controlled aseptic conditions. Before the procedure, the patient should be administered adequate anesthesia. The pupil of the eye to be injected should be dilated, and a broad-spectrum antibiotic should be administered topically according to standard medical practice before surgery.
Administration
Follow the steps described below to administer voretigene neparvovec to patients:
- Once Luxturna is diluted, it should be inspected visually before administration. If particles, turbidity, or discoloration are observed, the medicinal product should not be used.
- Connect the syringe containing the diluted product to the extension tube and the subretinal injection cannula. The product should be injected slowly through the extension tube and the subretinal injection cannula to eliminate any air bubbles in the system.
- The volume of product available for injection is confirmed in the syringe by aligning the tip of the plunger with the line marking 0.3 ml.
- After vitrectomy, Luxturna is administered via subretinal injection using a subretinal injection cannula introduced via the pars plana.
- Under direct visualization, the tip of the subretinal injection cannula is placed in contact with the surface of the retina. The recommended injection site should be located along the superior vascular arcade, at least 2 mm away from the center of the fovea. A small amount of product is slowly injected until an initial subretinal bleb is observed, and then the remaining volume is slowly injected until the total 0.3 ml is administered (Figure 1).
Figure 1: Tip of the subretinal injection cannula placed at the recommended injection site (surgeon's view)
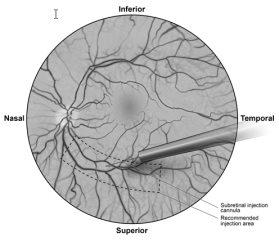
- After completion of the injection, the subretinal injection cannula is removed from the eye.
- After injection, any unused product should be discarded. The backup syringe should not be stored.
- Fluid-air exchange should be carefully performed, avoiding drainage of fluid near the retinotomy created for the subretinal injection.
- Postoperatively, the patient's head should be placed in a supine position immediately and maintained for 24 hours after discharge.
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to LUXTURNA 5 x 10¹² GENE VECTOR UNITS/ML CONCENTRATE AND SOLVENT FOR INJECTABLE SOLUTIONDosage form: EYEDROP, 5.5 mg sodium chloride; 3 mg hypromellose/mlActive substance: artificial tears and other indifferent preparationsManufacturer: Alcon Healthcare S.A.Prescription not requiredDosage form: EYEDROP, 3.2 mg/mlActive substance: artificial tears and other indifferent preparationsManufacturer: Bausch & Lomb S.A.Prescription not requiredDosage form: EYE DROP, 3.2 mg/mlActive substance: artificial tears and other indifferent preparationsManufacturer: Bausch & Lomb S.A.Prescription not required
Online doctors for LUXTURNA 5 x 10¹² GENE VECTOR UNITS/ML CONCENTRATE AND SOLVENT FOR INJECTABLE SOLUTION
Discuss questions about LUXTURNA 5 x 10¹² GENE VECTOR UNITS/ML CONCENTRATE AND SOLVENT FOR INJECTABLE SOLUTION, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions
















