
Corneregel

Ask a doctor about a prescription for Corneregel

How to use Corneregel
Package Leaflet: Information for the User
Warning! Keep the leaflet. Information on the immediate packaging in a foreign language.
Corneregel
50 mg/g (5%), eye gel
Dexpanthenolum
Read the leaflet carefully before using the medicine, as it contains important information for the patient.
- Keep this leaflet, so you can read it again if you need to.
- If you have any doubts, consult a doctor or pharmacist.
- This medicine has been prescribed specifically for you. Do not pass it on to others. The medicine may harm another person, even if their symptoms are the same.
- If the patient experiences any side effects, including any side effects not listed in this leaflet, they should tell their doctor, pharmacist, or nurse. See section 4.
Table of Contents of the Leaflet:
- 1. What is Corneregel and what is it used for
- 2. Important information before using Corneregel
- 3. How to use Corneregel
- 4. Possible side effects
- 5. How to store Corneregel
- 6. Contents of the pack and other information
1. What is Corneregel and what is it used for
The active substance of the medicine is dexpanthenol.
Corneregel is used to treat non-inflammatory keratopathy, e.g., corneal degeneration, corneal dystrophy, recurrent erosion, and damage related to contact lens use.
Additionally, as a supportive treatment for the healing process in corneal and conjunctival damage, chemical burns, and burns.
Supportively in specific treatments for bacterial, fungal, and viral corneal damage.
WARNING:
Corneregel is not intended to treat bacterial, fungal, and viral corneal damage, but only as a supportive treatment for the proper treatment of these corneal diseases.
2. Important information before using Corneregel
When not to use Corneregel
Warnings and precautions
- Before starting to use Corneregel, discuss it with your doctor or pharmacist. Corneregel contains cetrimide (a preservative), which may cause burning, redness, a feeling of a foreign body in the eye, and with frequent use, may damage the corneal epithelium.
- Do not wear contact lenses while using Corneregel, as the lenses may become discolored and there is a risk of incompatibility with the lens material. Remove contact lenses before instillation and do not put them back on until at least 10-15 minutes have passed.
Do not touch the dropper tip to the surface or area of the eye, or any other surface, as this may contaminate the dropper or gel with bacteria that can cause eye infections.
- Do not touch the dropper tip to the surface or area of the eye, or any other surface, as this may contaminate the dropper or gel with bacteria that can cause eye infections.
Corneregel and other medicines
Tell your doctor or pharmacist about all medicines you are taking, or have recently taken, and about any medicines you plan to take.
If Corneregel is used with other eye drops or ointments, keep a minimum of 15 minutes between administration of these medicines, and Corneregel should always be administered last.
Pregnancy, breastfeeding, and fertility
If you are pregnant or breastfeeding, think you may be pregnant, or plan to become pregnant, consult your doctor or pharmacist before using this medicine.
The medicine should not be used during pregnancy unless your doctor advises you to do so.
Driving and using machines
Corneregel, even when used correctly, may temporarily blur your vision due to the formation of streaks, which may affect your reaction ability. Do not drive, perform tasks without stable support, or operate machines until the symptoms have resolved.
3. How to use Corneregel
Always use this medicine exactly as your doctor or pharmacist has told you. If you are unsure, consult your doctor or pharmacist.
Use in the eye.
Recommended dose:
Depending on the severity and intensity of the disorders, 1 drop into the conjunctival sac 4 times a day, and 1 drop before bedtime.
There are no time limits for use. The product can be used until subjective symptoms have resolved.
Warning!
Administration instructions
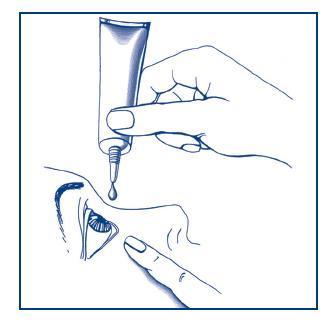
Wash your hands.
Tilt your head back and gently pull down the lower eyelid with your index finger.
With your other hand, position the tube vertically over the eye.
Do not touch the tube tip to the eye, eyelid, eye area, or any other surface.
This may lead to infection of the gel.Using infected gel can lead to serious complications and even vision loss.
Administer one drop into the conjunctival sac.
If the drop does not enter the eye, repeat the administration attempt.
After administration, try to keep the eye open and move it to distribute the gel evenly.
Overdose of Corneregel
Administration of a larger amount of the medicine than recommended is not associated with any additional risk.
Missed dose of Corneregel
Do not use a double dose to make up for a missed dose.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Very rare side effects (may affect up to 1 in 10,000 people): hypersensitivity reactions (e.g., itching, rash).
Side effects of unknown frequency include: eye irritation, e.g., redness, pain, feeling of a foreign body in the eye, tearing, eye itching, conjunctival edema.
Reporting side effects
If you experience any side effects, including any side effects not listed in the leaflet, tell your doctor, pharmacist, or nurse. Side effects can be reported directly to the Department of Monitoring of Adverse Reactions to Medicinal Products of the Office for Registration of Medicinal Products, Medical Devices, and Biocidal Products,
Al. Jerozolimskie 181 C
02-222 Warsaw
Phone: +48 22 49 21 301
Fax: + 48 22 49 21 309
Website: https://smz.ezdrowie.gov.pl
Reporting side effects will help gather more information on the safety of the medicine.
5. How to store Corneregel
Keep the medicine out of the sight and reach of children.
Do not store above 25°C.
Do not use this medicine after the expiry date stated on the outer packaging or immediate packaging. The expiry date refers to the last day of the specified month.
Discard any remaining contents 4 weeks after first opening the tube.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. This will help protect the environment.
6. Contents of the pack and other information
What Corneregel contains
- The active substance is dexpanthenol. 1 g of gel contains 50 mg of dexpanthenol.
- The other ingredients are: cetrimide, disodium edetate, sodium hydroxide, polyacrylic acid (Carbomer 940), water for injections.
What Corneregel looks like and contents of the pack
Corneregel is an eye gel. The medicine is supplied in tubes, in a cardboard box.
Pack sizes: 5 g and 10 g of gel.
For more detailed information, consult the marketing authorization holder or parallel importer.
Marketing authorization holder in Greece, the country of export:
Bausch + Lomb Ireland Limited
3013 Lake Drive
Citywest Business Campus 24
Dublin, Ireland
Manufacturer:
Dr. Gerhard Mann
Chem.-pharm. Fabrik GmbH
Brunsbutteler Damm 165-173
13581 Berlin
Germany
Parallel importer:
Delfarma Sp. z o.o.
ul. Św. Teresy od Dzieciątka Jezus 111
91-222 Łódź
Repackaged by:
Delfarma Sp. z o.o.
ul. Św. Teresy od Dzieciątka Jezus 111
91-222 Łódź
Authorization number in Greece, the country of export:
4768/5-2-2003
76459/21-12-2015
Parallel import authorization number: 118/08
Date of leaflet approval: 27.11.2023
[Information about the trademark]
- Country of registration
- Active substance
- Prescription requiredYes
- Marketing authorisation holder (MAH)Bausch + Lomb Ireland limited
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to CorneregelDosage form: Gel, 50 mg/gActive substance: dexpanthenolPrescription requiredDosage form: Gel, 50 mg/gActive substance: dexpanthenolPrescription requiredDosage form: Gel, 50 mg/gActive substance: dexpanthenolPrescription required
Alternatives to Corneregel in other countries
The best alternatives with the same active ingredient and therapeutic effect.
Alternative to Corneregel in Ukraine
Alternative to Corneregel in Spain
Online doctors for Corneregel
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for Corneregel – subject to medical assessment and local rules.














