

Berotec N 100

Ask a doctor about a prescription for Berotec N 100

How to use Berotec N 100
Leaflet attached to the packaging: patient information
Berotec N 100, 100 micrograms/dose, inhalation aerosol, solution
Fenoterol hydrobromide
Read the leaflet carefully before using the medicine, as it contains important information for the patient.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their symptoms are the same as yours.
- If you experience any side effects, including any not listed in this leaflet, please tell your doctor or pharmacist. See section 4.
Table of contents of the leaflet
- 1. What is Berotec N 100 and what is it used for
- 2. Important information before using Berotec N 100
- 3. How to use Berotec N 100
- 4. Possible side effects
- 5. How to store Berotec N 100
- 6. Contents of the pack and other information
1. What is Berotec N 100 and what is it used for
Berotec N 100 contains fenoterol, which belongs to a group of medicines called selective beta-adrenergic receptor agonists, which widen the airways and are used to treat asthma attacks and other conditions with reversible airway constriction.
The effect of Berotec N 100 occurs immediately after inhalation, so it can be used to treat acute asthma attacks.
Berotec N 100 is used to widen the airways in the treatment of acute asthma attacks and conditions with reversible airway constriction, especially in chronic obstructive pulmonary disease and to prevent exercise-induced asthma.
If the patient has asthma that responds to steroid treatment, the doctor may also prescribe anti-inflammatory medicines.
2. Important information before using Berotec N 100
When not to use Berotec N 100:
if you are allergic to fenoterol hydrobromide or any of the other ingredients of this medicine (listed in section 6),
if you have heart rhythm disorders with rapid heart rate,
if you have a heart disease called hypertrophic cardiomyopathy.
Warnings and precautions
Before starting to use Berotec N 100, you should discuss it with your doctor or pharmacist,
if you have recently had a heart attack;
if you have diabetes;
if you have heart and blood vessel disorders;
if you have severe heart disease and have experienced chest pain or other symptoms of worsening heart disease; in this case, you should contact your doctor immediately;
if you have a phaeochromocytoma (a type of adrenal gland tumor);
if you have hyperthyroidism;
if you are using other medicines from the group of sympathomimetics; the use of these medicines is possible only under close medical supervision. Other bronchodilator medicines from the group of parasympatholytics (e.g. tiotropium, ipratropium) can be used at the same time.
If any of the following situations occur, you should contact your doctor immediately:
- inhalation has caused airway constriction; in this case, you should stop using Berotec N 100 immediately, as further use may be life-threatening. Your doctor will prescribe other treatment.
- symptoms such as difficulty breathing or chest pain have occurred; you should consult your doctor to determine if they are due to heart or respiratory disease.
- symptoms such as loss of appetite, constipation, water retention or leg swelling, irregular heartbeat or muscle weakness have occurred. They may be due to decreased potassium levels in the blood, especially if Berotec N 100 is administered simultaneously with theophylline, corticosteroids or diuretics. Decreased potassium levels in the blood may increase the risk of heart rhythm disorders in patients treated with digoxin, as well as in patients with low oxygen levels in the blood. As a precaution, it may be necessary to perform appropriate tests, such as blood tests.
- acute, rapidly worsening shortness of breath has occurred.
- breathing difficulties do not improve or worsen. You should contact your doctor to verify the established treatment plan. Your doctor may consider adding other medicines. Never use a higher dose than prescribed, as this may lead to severe side effects.
If it is necessary to use Berotec N 100 for a longer period, your doctor will provide detailed instructions for use, as Berotec N 100 is usually used to treat acute symptoms, and not for regular use. Your doctor may also decide to prescribe additional anti-inflammatory medicines or increase their dose.
Using Berotec N 100 may lead to positive results for the presence of fenoterol in substance abuse tests, e.g. to increase performance in athletes (doping).
Berotec N 100 and other medicines
Tell your doctor about all medicines you are currently taking or have recently taken, as well as any medicines you plan to take.
Other bronchodilator medicines may enhance the effect of Berotec N 100.
Concomitant administration of other beta-adrenergic receptor stimulants, oral anticholinergics and xanthine derivatives (e.g. theophylline) may also increase the frequency of side effects.
Using Berotec N 100 with other medicines, such as theophylline, corticosteroids or diuretics, may further decrease potassium levels in the blood, see section "Warnings and precautions".
Certain medicines used to treat high blood pressure (so-called beta-adrenergic receptor blockers) may weaken the effect of Berotec N 100.
Tell your doctor if you are taking certain antidepressants (monoamine oxidase inhibitors (MAO) or tricyclic antidepressants). You should be cautious when using such medicines, as they may enhance the effect of Berotec N 100.
Inhalation of halogenated general anesthetics, such as halothane, trichloroethylene and enflurane, may enhance the effect of Berotec N 100 (e.g. may cause irregular heartbeat).
Pregnancy, breastfeeding and fertility
If you are pregnant or breastfeeding, think you may be pregnant or are planning to have a baby, ask your doctor or pharmacist for advice before using this medicine.
Non-clinical studies and human experience have not shown any adverse effects of fenoterol hydrobromide during pregnancy. However, Berotec N 100 should only be used during pregnancy if your doctor considers it necessary. This is especially true during the first trimester of pregnancy and immediately before delivery (Berotec N 100 inhibits uterine contractions).
Non-clinical studies have shown that fenoterol hydrobromide passes into breast milk. Therefore, Berotec N 100 should only be used during breastfeeding if your doctor considers it necessary.
Driving and using machines
No studies have been conducted on the effect of the medicine on the ability to drive and use machines. However, during use, side effects such as dizziness may occur. Therefore, if these side effects occur, you should avoid potentially hazardous activities such as driving and using machines.
The medicine contains ethanol and sodium
This medicine contains 15.597 mg of alcohol (ethanol) in each puff. The amount of alcohol in each puff of this medicine is equivalent to less than 1 ml of beer or 1 ml of wine. The small amount of alcohol in this medicine will not have noticeable effects.
The medicine contains less than 1 mmol (23 mg) of sodium per dose, which means that the medicine is considered "sodium-free".
3. How to use Berotec N 100
This medicine should always be used as directed by your doctor. If you are unsure, ask your doctor or pharmacist.
Berotec N 100 should rather be used as needed, and not regularly.
You should seek medical help immediately if your asthma symptoms (cough, shortness of breath, wheezing or chest tightness) worsen or if you experience shortness of breath that prevents you from speaking, eating or sleeping.
Using Berotec N 100 more frequently than twice a week to treat asthma symptoms, in addition to preventive use before physical exertion, indicates poorly controlled asthma and may increase the risk of severe asthma attacks (asthma exacerbation), which can lead to serious complications and can be life-threatening or even fatal. You should contact your doctor as soon as possible to verify your asthma treatment.
If you are using a medicine to prevent inflammation in the lungs, e.g. an "inhalation corticosteroid", it is important to continue using it regularly, even if you feel better.
Recommended dose
Your doctor will adjust the dosage individually for each patient. If not otherwise prescribed, the following dosage is used for adults and children over 6 years of age:
a) Symptomatic treatment of acute asthma attacks and other conditions with reversible airway constriction
One dose (one puff) of the aerosol is sufficient to relieve symptoms in most cases. In more severe cases, if no significant improvement in breathing is observed after 5 minutes, an additional dose can be used, but no more than 8 puffs of the aerosol per day.
If the asthma attack does not subside after 2 puffs of the aerosol, it may be necessary to use a larger number of puffs. In this case, you should contact your doctor or go to the nearest hospital immediately.
b) Prevention of exercise-induced asthma
- 1 - 2 puffs before physical exertion, up to a maximum of 8 puffs of the aerosol per day.
Use in children and adolescents
In children, the Berotec N 100 inhalation aerosol should only be used on the advice of a doctor and under adult supervision.
Instructions for use
The Berotec N 100 inhalation aerosol should be used as described below.
Before first use, press the dosing valve on the container twice.
During each use of the product, follow these steps:
- 1. Remove the protective cap.
- 2. Take a deep breath out.
- 3. Hold the container with the aerosol in the position shown in Fig. 1, with your lips tightly closed around the mouthpiece. The arrow on the container and the bottom of the container should be pointing upwards.
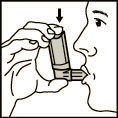
(Fig. 1)
- 4. Take a deep breath in, while firmly pressing the bottom of the container, which will release one measured dose of the medicine. Hold your breath for a few seconds, then remove the mouthpiece from your mouth and breathe out. Repeat these steps (points 2-4) if you need to take a second dose of the medicine.
- 5. After use, replace the protective cap.
- 6. If you do not use the aerosol for a period of three days, press the dosing valve once before reuse.
The container is not transparent, so you cannot see if it is empty. A new container provides 200 puffs containing 100 micrograms of fenoterol hydrobromide. After using this number of puffs, a small amount of solution may still be left in the container. However, you should replace the container with a new one to ensure that each puff delivers the correct amount of medicine.
Always keep the mouthpiece clean and wash it in warm water. If you use soap or detergent, rinse the mouthpiece thoroughly with clean water.
The inhaler mouthpiece should be washed at least once a week. It is important to keep the mouthpiece clean to ensure that the medicine does not accumulate on the walls of the mouthpiece and block the inhaler.
To wash the inhaler mouthpiece, first remove the protective cap and take the medicine container out of the mouthpiece. Rinse the mouthpiece with warm water until all visible impurities are removed.
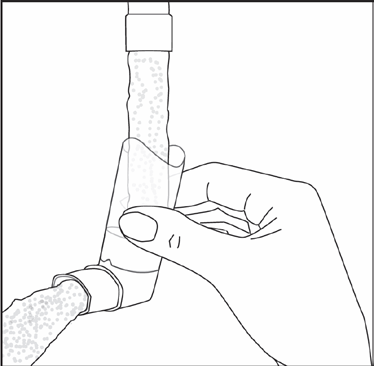
(Fig. 2)
After washing, shake the inhaler mouthpiece and leave it to air dry. Do not use any drying systems. When the inhaler mouthpiece is dry, put the medicine container back into the mouthpiece and replace the protective cap.
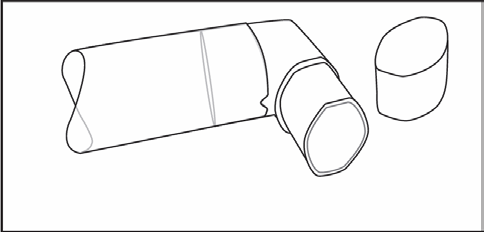
(Fig. 3)
WARNING:
The plastic mouthpiece is designed specifically for use with the Berotec N 100 inhalation aerosol to ensure that the correct dose of medicine is always delivered. The mouthpiece should never be used with any other inhalation aerosol, and the Berotec N 100 inhalation aerosol should not be used with any other mouthpiece than the one provided with the product.
The container is under pressure and should not be opened by force or exposed to temperatures above 50°C.
Using a higher dose of Berotec N 100 than recommended
If you have used a higher dose of the medicine than recommended, consult your doctor or pharmacist.
Expected symptoms of overdose are related to excessive effects on the cardiovascular system. They include side effects listed in section 4, in particular:
rapid heart rate, palpitations (feeling of strong heartbeats), tremors, changes in blood pressure, decreased diastolic and increased systolic blood pressure, chest pain, irregular heartbeat and flushing of the face. Increased acidity of the blood (metabolic acidosis) and decreased potassium levels in the blood have also been observed during the use of fenoterol in doses higher than recommended for Berotec N 100.
Missing a dose of Berotec N 100
Do not use a double dose to make up for a missed dose.
Stopping use of Berotec N 100
If you stop using Berotec N 100, your breathing problems may return or worsen. Therefore, you should use Berotec N 100 for as long as your doctor has prescribed. In other cases, consult your doctor before stopping use of Berotec N 100.
If you have any further questions about the use of this medicine, ask your doctor or pharmacist.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
The following side effects have been reported during clinical trials and post-marketing surveillance of Berotec N 100:
Common(occurring in less than 1 in 10 people):
- tremors
- cough
Uncommon(occurring in less than 1 in 100 people):
- inhalation-induced airway constriction
- decreased potassium levels in the blood, including severe cases
- excitement
- heart rhythm disorders
- nausea
- vomiting
- itching
Frequency not known(frequency cannot be estimated from available data):
- hypersensitivity reactions
- nervousness
- headache
- dizziness
- rapid heartbeat
- palpitations
- myocardial ischemia
- throat irritation
- excessive sweating
- skin reactions, such as rash, urticaria
- muscle pain
- muscle cramps
- muscle weakness
- decreased diastolic and increased systolic blood pressure
Reporting side effects
If you experience any side effects, including any not listed in this leaflet, please tell your doctor, pharmacist or nurse. Side effects can be reported directly to the Department of Drug Safety Monitoring of the Office for Registration of Medicinal Products, Medical Devices and Biocidal Products: Al. Jerozolimskie 181C, 02-222 Warsaw, tel.: + 48 22 49-21-301, fax: + 48 22 49-21-309, website: https://smz.ezdrowie.gov.pl.
Side effects can also be reported to the marketing authorization holder. By reporting side effects, you can help provide more information on the safety of this medicine.
5. How to store Berotec N 100
Keep the medicine out of the sight and reach of children.
There are no special precautions for storage.
Do not use this medicine after the expiry date stated on the packaging.
The expiry date refers to the last day of the month stated.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines that are no longer needed. This will help protect the environment.
6. Contents of the pack and other information
What Berotec N 100 contains
- The active substance of the medicine is fenoterol hydrobromide. One dose of the inhalation aerosol contains 100 micrograms of fenoterol hydrobromide.
- The other ingredients of the medicine are: 1,1,1,2-tetrafluoroethane (HFA 134a), anhydrous citric acid, purified water, anhydrous ethanol.
This medicine contains fluorinated greenhouse gases.
Each inhaler contains 9.17 g of HFA 134a (1,1,1,2-tetrafluoroethane), which corresponds to 0.01311 tons of CO2 equivalent (global warming potential GWP = 1430).
What Berotec N 100 looks like and contents of the pack
Berotec N 100 is available in a stainless steel container with a capacity of 17 ml, containing 10 ml of solution, with a dosing valve and a plastic mouthpiece in a cardboard box.
Pack size: 1 container of 10 ml (200 doses).
Marketing authorization holder and manufacturer
Marketing authorization holder:
Boehringer Ingelheim International GmbH
Binger Strasse 173
D-55216 Ingelheim/Rhein
Germany
Manufacturer:
Boehringer Ingelheim Pharma GmbH & Co. KG
Binger Strasse 173
D-55216 Ingelheim/Rhein
Germany
To obtain more detailed information about this medicine, please contact the local representative of the marketing authorization holder:
Poland
Boehringer Ingelheim Sp. z o.o.
Tel.: +48 22 699 0 699
Date of last revision of the leaflet:
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- ImporterBoehringer Ingelheim Pharma KG
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to Berotec N 100Dosage form: Powder, 50 mcg/dose inh.Active substance: salmeterolManufacturer: Polfarmex S.A.Prescription requiredDosage form: Aerosol, 100 mcg/dose inh.Active substance: salbutamolManufacturer: Laboratorio Aldo-Union S.A.Prescription requiredDosage form: Aerosol, 12 mcg/measured doseActive substance: formoterolManufacturer: Chiesi Farmaceutici S.p.A. Chiesi Pharmaceuticals GmbHPrescription required
Alternatives to Berotec N 100 in other countries
The best alternatives with the same active ingredient and therapeutic effect.
Alternative to Berotec N 100 in Ukraine
Alternative to Berotec N 100 in Spain
Online doctors for Berotec N 100
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for Berotec N 100 – subject to medical assessment and local rules.














