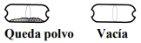OSLIF BREEZHALER 150 micrograms INHALATION POWDER (HARD CAPSULE)

How to use OSLIF BREEZHALER 150 micrograms INHALATION POWDER (HARD CAPSULE)
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the User
Oslif Breezhaler 150 micrograms inhalation powder (hard capsule)
Oslif Breezhaler 300 micrograms inhalation powder (hard capsule)
indacaterol
Read this entire leaflet carefully before you start using this medicine, because it contains important information for you.
- Keep this leaflet, as you may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their symptoms are the same as yours.
- If you experience any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the pack and other information
- What Oslif Breezhaler is and what it is used for
- What you need to know before you use Oslif Breezhaler
- How to use Oslif Breezhaler
- Possible side effects
- Storage of Oslif Breezhaler
Contents of the pack and additional information
1. What Oslif Breezhaler is and what it is used for
What Oslif Breezhaler is
Oslif Breezhaler contains the active substance indacaterol, which belongs to a group of medicines called bronchodilators. When inhaled, it relaxes the muscles of the walls of the small airways in the lungs. This helps to open up the airways, making it easier to breathe in and out.
What Oslif Breezhaler is used for
Oslif Breezhaler is used to help adults who have difficulty breathing due to a lung disease called chronic obstructive pulmonary disease (COPD). In COPD, the muscles surrounding the airways contract, making it difficult to breathe. This medicine relaxes these muscles in the lungs, making it easier to breathe in and out.
2. What you need to know before you use Oslif Breezhaler
Do not use Oslif Breezhaler
- if you are allergic to indacaterol or any of the other ingredients of this medicine (listed in section 6).
Warnings and precautions
Talk to your doctor or pharmacist before you start using Oslif Breezhaler
- if you have asthma (in this case, you should not use Oslif Breezhaler).
- if you have heart problems.
- if you have epilepsy.
- if you have problems with your thyroid gland (thyrotoxicosis).
- if you have diabetes.
During treatment with Oslif Breezhaler,
- Stop using the medicine and tell your doctor immediatelyif you notice chest tightness, coughing, wheezing, or difficulty breathing immediately after using the medicine. These may be signs of a condition called bronchospasm.
- Tell your doctor immediatelyif your COPD symptoms (difficulty breathing, wheezing, coughing) do not improve or worsen.
Children and adolescents
Oslif Breezhaler must notbe given to children or adolescents under 18 years of age.
Other medicines and Oslif Breezhaler
Tell your doctor or pharmacist if you are using, have recently used, or might use any other medicines.
Especially, tell your doctor if you are using:
- medicines for respiratory problems that are similar to Oslif Breezhaler (i.e., medicines such as salmeterol and formoterol). You may be more likely to have side effects.
- medicines called beta-blockers that are used for high blood pressure or other heart problems (such as propranolol), or for a problem with the eyes called glaucoma (such as timolol).
- medicines that decrease the amount of potassium in the blood. These include:
- corticosteroids (e.g., prednisolone),
- diuretics used for high blood pressure, such as hydrochlorothiazide,
- medicines for respiratory problems such as theophylline.
Pregnancy and breastfeeding
If you are pregnant or breastfeeding, think you may be pregnant, or are planning to have a baby, ask your doctor or pharmacist for advice before using this medicine.
Do not use Oslif Breezhaler unless your doctor tells you to.
Ask your doctor or pharmacist for advice before using any medicine.
Driving and using machines
Oslif Breezhaler is unlikely to affect your ability to drive or use machines.
Oslif Breezhaler contains lactose
This medicine contains lactose (milk sugar). If your doctor has told you that you have an intolerance to some sugars, contact them before taking this medicine.
3. How to use Oslif Breezhaler
Follow the instructions for administration of this medicine exactly as your doctor has told you. If you are unsure, ask your doctor or pharmacist again.
How much Oslif Breezhaler to use
- The usual dose is to inhale the contents of one capsule each day. Your doctor will tell you whether to use the 150 microgram or 300 microgram capsule, depending on your condition and how you respond to treatment. Do not use more than the dose your doctor has prescribed.
- Use your inhaler at the same time each day. The effects last for 24 hours. This will ensure that you always have enough medicine in your body to help you breathe more easily throughout the day and night. It will also help you remember to use it.
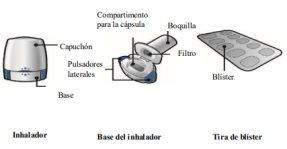
How to use Oslif Breezhaler
- In this pack, you will find an inhaler and capsules (in a blister pack) that contain the medicine in powder form for inhalation. The Oslif Breezhaler inhaler will allow you to inhale the medicine contained in one capsule.
- Use the capsules only with the inhaler provided in this pack (Oslif Breezhaler inhaler). The capsules should be kept in the blister pack until you need to use them.
- When you start a new pack, use the new Oslif Breezhaler inhaler provided in the pack.
- Discard the inhaler once all the capsules have been used.
- Do not swallow the capsules.
- For more information on how to use the inhaler, read the instructions at the end of this leaflet.
If you use more Oslif Breezhaler than you should
If you have inhaled too much Oslif Breezhaler or if someone uses your capsules, tell your doctor immediately or go to the nearest emergency room. Show the Oslif Breezhaler pack. Medical attention may be necessary. You may notice that your heart beats faster than normal, or you may have a headache, feel drowsy, nauseous, or vomit.
If you forget to use Oslif Breezhaler
If you forget to inhale a dose, inhale one at the usual time the next day. Do not inhale a double dose to make up for forgotten doses.
How long to continue treatment with Oslif Breezhaler
- Continue using your treatment with Oslif Breezhaler for as long as your doctor tells you.
- COPD is a long-term disease, and you should use Oslif Breezhaler every dayand not just when you have breathing problems or other COPD symptoms.
Ask your doctor or pharmacist if you have any doubts about how long to continue treatment with Oslif Breezhaler.
If you have any further questions on the use of this medicine, ask your doctor or pharmacist.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Some side effects may be serious. Tell your doctor immediately
- if you have chest pain (common).
- if you have high blood sugar levels (diabetes). You will feel tired, very thirsty, and hungry (without gaining weight), and you will urinate more than usual (common).
- if you have irregular heartbeats (uncommon).
- if you have symptoms of an allergic reaction such as skin rash, itching, hives, difficulty breathing or swallowing, dizziness (uncommon).
- if you have difficulty breathing with wheezing or coughing (uncommon).
Other side effects may include:
Very common side effects (may affect more than 1 in 10 people)
- flu-like symptoms. You may have all or most of the following effects: sore throat, runny nose, blocked nose, sneezing, cough, headache.
Common side effects (may affect up to 1 in 10 people)
- feeling of pain or pressure in the cheeks and forehead (sinusitis)
- runny nose
- cough
- sore throat
- headache
- dizziness
- palpitations
- muscle spasms
- swelling of the hands, ankles, and feet (edema)
- itching/skin rash
- chest pain
- pain in the muscles, bones, or joints
Uncommon side effects (may affect up to 1 in 100 people)
- fast heartbeat
- tingling or numbness
- muscle pain
Some people may occasionally cough shortly after inhaling the medicine. Coughing is a common symptom in COPD. If you experience coughing shortly after inhaling the medicine, do not worry. Check your inhaler to see if the capsule is empty and that you have received the full dose. If the capsule is empty, there is no need to worry. If the capsule is not empty, inhale again as instructed.
Reporting of side effects
If you experience any side effects, talk to your doctor, pharmacist, or nurse. This includes any possible side effects not listed in this leaflet. You can also report side effects directly through the national reporting system listed in Appendix V. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of Oslif Breezhaler
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date which is stated on the carton and blister after “EXP”. The expiry date is the last day of the month stated.
Do not store above 30°C.
Store in the original package to protect from moisture and do not remove until immediately before use.
Do not use this medicine if you notice that the pack is damaged or shows signs of deterioration.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. This will help protect the environment.
6. Contents of the pack and additional information
Composition of Oslif Breezhaler
- Each 150 microgram capsule of Oslif Breezhaler contains 150 micrograms of indacaterol as indacaterol maleate. The other ingredients include lactose and the gelatin capsule.
- Each 300 microgram capsule of Oslif Breezhaler contains 300 micrograms of indacaterol as indacaterol maleate. The other ingredients include lactose and the gelatin capsule.
Appearance of Oslif Breezhaler and pack contents
In this pack, you will find an inhaler, along with capsules in a blister pack. The capsules are transparent (colorless) and contain a white powder.
- The 150 microgram capsules of Oslif Breezhaler have a product code “IDL 150” printed in blackabove a blackline and the company logo () printed in blackbelow it.
- The 300 microgram capsules of Oslif Breezhaler have a product code “IDL 300” printed in blueabove a blueline and the company logo () printed in bluebelow it.
The following pack sizes are available:
Carton containing 10 capsules and 1 inhaler.
Carton containing 30 capsules and 1 inhaler.
Multiple pack containing 2 packs (each containing 30 capsules and 1 inhaler).
Multiple pack containing 3 packs (each containing 30 capsules and 1 inhaler).
Multiple pack containing 30 packs (each containing 10 capsules and 1 inhaler).
Only some pack sizes may be marketed.
Marketing authorisation holder
Novartis Europharm Limited
Vista Building
Elm Park, Merrion Road
Dublin 4
Ireland
Manufacturer
Novartis Pharma GmbH
Roonstraße 25
D-90429 Nuremberg
Germany
Novartis Farmacéutica SA
Gran Via de les Corts Catalanes, 764
08013 Barcelona
Spain
Novartis Pharma GmbH
Sophie-Germain-Strasse 10
90443 Nürnberg
Germany
You can request more information about this medicine by contacting the local representative of the marketing authorisation holder:
Belgium Novartis Pharma N.V. Tel: +32 2 246 16 11 | Lithuania SIA Novartis Baltics Lietuvos filialas Tel: +370 5 269 16 50 |
| Luxembourg Novartis Pharma N.V. Tel: +32 2 246 16 11 |
Czech Republic Novartis s.r.o. Tel: +420 225 775 111 | Hungary Novartis Hungária Kft. Tel.: +36 1 457 65 00 |
Denmark Novartis Healthcare A/S Tlf: +45 39 16 84 00 | Malta Novartis Pharma Services Inc. Tel: +356 2122 2872 |
Germany Novartis Pharma GmbH Tel: +49 911 273 0 | Netherlands Novartis Pharma B.V. Tel: +31 88 04 52 111 |
Estonia SIA Novartis Baltics Eesti filiaal Tel: +372 66 30 810 | Norway Novartis Norge AS Tlf: +47 23 05 20 00 |
Greece Novartis (Hellas) A.E.B.E. Τηλ: +30 210 281 17 12 | Austria Novartis Pharma GmbH Tel: +43 1 86 6570 |
Spain Ferrer Internacional, S.A. Tel: +34 93 600 37 00 | Poland Novartis Poland Sp. z o.o. Tel.: +48 22 375 4888 |
France Pierre Fabre Médicament Tél: +33 1 49 10 96 18 | Portugal Laboratório Medinfar - Produtos Farmacêuticos, S.A. Tel: +351 21 499 7400 |
Croatia Novartis Hrvatska d.o.o. Tel. +385 1 6274 220 | Romania Novartis Pharma Services Romania SRL Tel: +40 21 31299 01 |
Ireland Novartis Ireland Limited Tel: +353 1 260 12 55 | Slovenia Novartis Pharma Services Inc. Tel: +386 1 300 75 50 |
Iceland Vistor hf. Sími: +354 535 7000 | Slovakia Novartis Slovakia s.r.o. Tel: +421 2 5542 5439 |
Italy Novartis Farma S.p.A. Tel: +39 02 96 54 1 | Finland Novartis Finland Oy Puh/Tel: +358 (0)10 6133 200 |
Cyprus Novartis Pharma Services Inc. Τηλ: +357 22 690 690 | Sweden Novartis Sverige AB Tel: +46 8 732 32 00 |
Latvia SIA Novartis Baltics Tel: +371 67 887 070 |
Date of last revision of this leaflet:
Other sources of information
Detailed information on this medicine is available on the European Medicines Agency website: http://www.ema.europa.eu
INSTRUCTIONS FOR USE OF OSLIF BREEZHALER INHALER
Read the Instructions for Usecompletely before using Oslif Breezhaler.
Insert |
Puncture and release |
Inhale deeply |
Check that the capsule is empty |
|
|
|
|
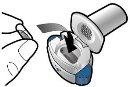
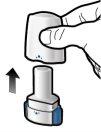
Step 1a: Remove the cap.
Step 1b: Open the inhaler
Step 1c: Remove the capsule Remove one capsule from the blister pack. Do not swallow the capsule. |
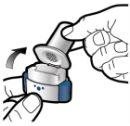
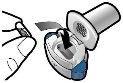
Step 2a: Puncture the capsule once Hold the inhaler in a vertical position. Puncture the capsule by firmly pressing both buttons at the same time. You should hear a noise when the capsule is punctured. Puncture the capsule only once.
Step 2b: Release the buttons completely |
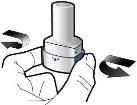
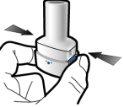
Step 3a: Exhale completely Do not blow into the inhaler.
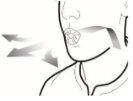
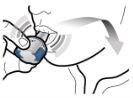
Step 3b: Inhale the medication deeply Hold the inhaler as shown in the figure. Insert the mouthpiece into your mouth and close your lips firmly around it. Do not press the buttons Inhale quickly and as deeply as possible. During inhalation, you will hear a humming noise. You may notice the taste of the medication when you inhale.
Step 3c: Hold your breath Hold your breath for 5 seconds. |
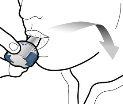
Check that the capsule is empty Open the inhaler to check if there is any powder left in the capsule. If there is powder left in the capsule:
Remove the empty capsule Discard the empty capsule in your household trash. Close the inhaler and replace the cap. |
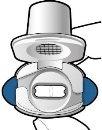
Step 1d: Insert the capsule Never place the capsule directly into the mouthpiece.
Step 1e: Close the inhaler | Important information
|
Your Oslif Breezhaler pack contains:
| Frequently asked questions Why did the inhaler not make a noise when I inhaled? The capsule may be stuck in the compartment. If this happens, carefully release the capsule by tapping the base of the inhaler. Inhale the medication again, repeating steps 3a to 3c. What should I do if there is powder left in the capsule? You have not received enough of your medication. Close the inhaler and repeat steps 3a to 3c. I coughed after inhaling, is it important? It may happen. If the capsule is empty, it means you have received enough of your medication. I notice small fragments of the capsule on my tongue, is it important? It may happen. It is not harmful. The likelihood of the capsules breaking into fragments increases if the capsule is punctured more than once. | Cleaning the inhaler Wipe the mouthpiece from the inside and outside with a clean, dry cloth that does not leave lint to remove any powder residue. Keep the inhaler dry. Never wash your inhaler with water. |
Disposal of the inhaler after use Each inhaler should be discarded after all the capsules have been used. Ask your pharmacist how to dispose of medicines and inhalers that are no longer needed. |
- Country of registration
- Average pharmacy price50.13 EUR
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to OSLIF BREEZHALER 150 micrograms INHALATION POWDER (HARD CAPSULE)Dosage form: PULMONARY INHALATION, 150 µgActive substance: indacaterolManufacturer: Novartis Europharm LimitedPrescription requiredDosage form: PULMONARY INHALATION, 300 µgActive substance: indacaterolManufacturer: Novartis Europharm LimitedPrescription requiredDosage form: PULMONARY INHALATIONActive substance: indacaterolManufacturer: Novartis Europharm LimitedPrescription required
Online doctors for OSLIF BREEZHALER 150 micrograms INHALATION POWDER (HARD CAPSULE)
Discuss questions about OSLIF BREEZHALER 150 micrograms INHALATION POWDER (HARD CAPSULE), including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions