
Berinert 1500

Ask a doctor about a prescription for Berinert 1500

How to use Berinert 1500
PATIENT INFORMATION LEAFLET
Berinert 1500
1500 IU
powder and solvent for solution for injection
Human C1-esterase inhibitor
Read the leaflet carefully before using the medicine.
- You should keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you, do not pass it on to others. It may harm them, even if their symptoms are the same as yours.
- If you experience any side effects, including those not listed in this leaflet, please inform your doctor or pharmacist.
Table of contents of the leaflet:
- 1. What is Berinert and what is it used for
- 2. Important information before using Berinert
- 3. How to use Berinert
- 4. Possible side effects
- 5. How to store Berinert
- 6. Contents of the pack and other information
1. WHAT IS BERINERT AND WHAT IS IT USED FOR
What is Berinert
Berinert is a medicine that comes in the form of a powder and solvent. After reconstitution, it is administered intravenously.
Berinert is produced from human plasma (the liquid part of the blood). Its active ingredient is a protein - human C1-esterase inhibitor.
What is Berinert used for
Berinert is used to treat and prevent hereditary angioedema (HAE) type I and II (swelling = edema). Hereditary angioedema is a congenital blood vessel disorder. It is not an allergic disease. It is caused by a deficiency, lack, or disorder of the synthesis of an important protein called C1-esterase inhibitor.
The disease is characterized by the following symptoms:
- sudden swelling of the hands and feet,
- sudden swelling of the face with a feeling of tension,
- swelling of the eyelids, lips, swelling of the larynx with difficulty breathing,
- swelling of the tongue,
- severe abdominal pain. In principle, swelling can affect any part of the body.
2. IMPORTANT INFORMATION BEFORE USING BERINERT
This section of the leaflet contains information that you should consider before starting treatment with Berinert.
When not to use Berinert:
- if you are allergic to C1-esterase inhibitor or any of the other ingredients of this medicine (listed in section 6).
Tell your doctor or pharmacist if you are sensitive to any medicine or food.
Warnings and precautions:
- if you have had allergic reactions to Berinert in the past. As a precaution, take antihistamines and corticosteroids.
- if you experience allergic symptoms or anaphylactic reaction (severe allergic symptoms can cause significant breathing difficulties or dizziness). Administration of
Berinert must be stopped immediately (i.e., the injection must be interrupted).
- if you have had laryngeal edema. You should be closely monitored in a place where immediate medical intervention is possible (hospital).
- if the medicine is used inconsistently with indications and dosage (e.g., capillary leak syndrome - CLS). See section 4. "Possible side effects".
Always consider the benefits and risks of using Berinert.
Virus safety
When medicines are produced from human blood or plasma, various measures are taken to protect the patient from the transmission of infectious agents. These methods include:
- proper selection of blood and plasma donors to exclude the risk of transmitting infectious agents
- testing each donation and plasma pool for infection markers.
Manufacturers of such medicines use methods of inactivation or removal of viruses during production.
Despite the use of the above methods, it is not possible to completely exclude the possibility of transmitting infectious agents after administering a medicinal product derived from human blood or plasma.
This risk also applies to unknown or newly discovered viruses and other infectious agents.
The measures taken are effective against enveloped viruses such as human immunodeficiency virus (HIV, the virus that causes AIDS), hepatitis B virus (HBV, which causes hepatitis B), hepatitis C virus (HCV, which causes hepatitis C), and non-enveloped viruses such as hepatitis A virus (HAV, which causes hepatitis A) and parvovirus B 19.
In patients who repeatedly receive products derived from human plasma, vaccination against viral hepatitis A and B should be considered.
Each time Berinert is administered, the administration date, batch number, and administered volume should be recorded in the patient's medical records.
Berinert and other medicines
- Tell your doctor or pharmacist about any other medicines you are taking, including those available without a prescription.
- Berinert must not be mixed in a syringe with other medicinal products or with other solvents.
Pregnancy and breastfeeding
- If you are pregnant or breastfeeding, think you may be pregnant, or plan to have a child, consult your doctor or pharmacist before using this medicine.
- During pregnancy and breastfeeding, Berinert should only be used if clearly necessary.
Driving and using machines
No studies have been conducted on the effects on the ability to drive and use machines.
Important information about some ingredients of Berinert
Berinert 1500 contains less than 1 mmol (23 mg) of sodium per vial, which means the medicine is considered "sodium-free".
3. HOW TO USE BERINERT
Treatment should be started and supervised by a doctor who has experience in using C1-esterase inhibitor in patients with deficiencies of this protein.
Dosage
Adults
Treatment of acute angioedema attack:
20 IU/kg body weight (20 IU/kg bw)
Preoperative prophylaxis of angioedema:
1000 IU less than 6 hours before medical, dental, or surgical procedure.
Children and adolescents
Treatment of acute angioedema attack:
20 IU/kg body weight (20 IU/kg bw)
Preoperative prophylaxis of angioedema:
15 to 30 IU/kg body weight (15-30 IU/kg bw) less than 6 hours before medical, dental, or surgical procedure. The dose should be chosen based on clinical circumstances (e.g., type of procedure and severity of the disease).
Overdose
No cases of overdose have been reported.
Reconstitution and administration
Berinert is usually administered intravenously by a nurse or doctor. The patient or their caregiver can also administer the injection, but only after proper training. If the doctor decides that the patient can undergo home therapy, they will provide detailed instructions. The patient will need to keep a treatment diary to document each injection performed at home. This diary should be taken to each doctor's visit. Regular verification of the patient's injection technique will be performed to ensure continuity of proper administration.
General instructions
- The powder should be reconstituted and withdrawn from the vial in aseptic conditions. The syringe provided with the product should be used.
- The prepared solution should be colorless and clear to slightly opalescent. After filtration or withdrawal from the vial (see below) and before administration, the solution should be carefully inspected for any impurities or sediment and for any change in color.
- If the solution is cloudy or visible particles are present, do not administer the reconstituted medicine.
- Unused medicine and any unused component of the kit should be disposed of in accordance with applicable regulations. Disposal instructions should be provided by the treating physician.
Method of reconstitution
Without opening the vials, warm the Berinert powder and solvent to room temperature.
This can be achieved by leaving the vials at room temperature for 1 hour or by holding them in your hands for a few minutes.
DO NOTheat the vials directly. The vials should not be heated above body temperature (37°C).
Carefully remove the caps from the vials containing the powder and solvent.
Wipe the injection site on the stopper with an alcohol swab (each vial with a separate swab) and let it dry.
The solvent can be combined with the powder using the provided Mix2Vial adapter.
Follow the instructions below.
1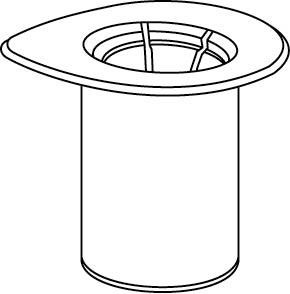 |
|
2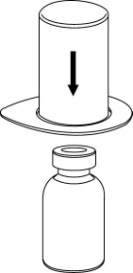 |
|
3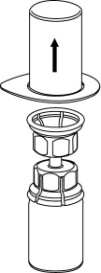 |
|
4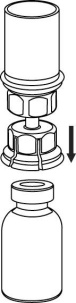 |
|
5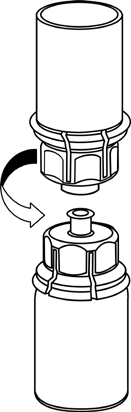 |
|
6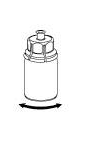 |
|
7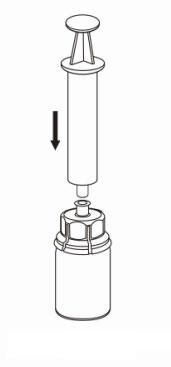 |
|
Withdrawal and administration
8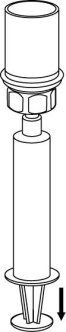 |
|
9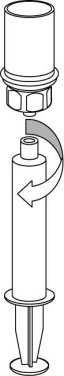 |
|
Administration
The prepared solution should be administered slowly intravenously.
4. POSSIBLE SIDE EFFECTS
Like all medicines, Berinert can cause side effects, although not everybody gets them.
Seek medical attention immediately if:
- you experience any side effect or
- you experience any symptom not listed in this leaflet.
In most patients, side effects after administration of Berinert are rare.
Rarely, the following side effects have been observed (may affect up to 1 in 1,000 patients):
- During prophylaxis or treatment of patients with capillary leak syndrome (fluid leakage from small vessels into tissue), there is a risk of increased blood clot formation, e.g., during or after cardiac surgery using extracorporeal circulation. See section 2. "Warnings and precautions".
- Increased body temperature, as well as a burning sensation and tingling at the injection site.
- Hypersensitivity or allergic reactions, such as: irregular heartbeat, rapid heartbeat, decreased blood pressure, redness of the skin, feeling of heat and redness of the face, difficulty breathing, pain, and dizziness, nausea.
Very rarely (may affect up to 1 in 10,000 patients or single cases), hypersensitivity reactions can lead to shock.
Reporting side effects
If you experience any side effects, including those not listed in this leaflet, please inform your doctor or pharmacist. Side effects can be reported directly to the Department of Drug Safety Monitoring of the Office for Registration of Medicinal Products, Medical Devices, and Biocidal Products
Al. Jerozolimskie 181C
02-222 Warsaw
tel.: +48 22 49 21 301
fax: +48 22 49 21 309
Website: https://smz.ezdrowie.gov.pl
By reporting side effects, you can help provide more information on the safety of this medicine.
Side effects can also be reported to the marketing authorization holder.
5. HOW TO STORE BERINERT
- Keep the medicine out of the sight and reach of children.
- Do not use Berinert after the expiry date stated on the label and outer packaging after EXP.
- Do not store above 30°C.
- Do not freeze.
- Store the vial in the outer packaging to protect it from light.
- Berinert does not contain preservatives, and after reconstitution, it should be used immediately.
- If the solution is not used immediately after preparation, it should be administered no later than 8 hours after preparation and should be stored only in the syringe.
The batch number of the product on the packaging with the administration set, outer packaging, and immediate packaging is stated after the abbreviation: Lot.
6. OTHER INFORMATION
What contains Berinert Active substance:
Human C1-esterase inhibitor (1500 IU/vial; after reconstitution in 3 ml water for injection: 500 IU/ml).
See the section "Information intended for healthcare professionals only" for more detailed information.
Excipients:
Glycine, sodium chloride, sodium citrate.
See the last paragraph of section 2. "Important information about some ingredients of Berinert".
Solvent:Water for injection
What Berinert looks like and contents of the pack
Berinert comes in the form of a white powder and solvent, which is water for injection.
The reconstituted solution should be colorless and clear to slightly opalescent.
Pack sizes
The pack with 1500 IU contains:
1 vial with powder (1500 IU)
1 vial with 3 ml water for injection
1 transfer system with a 20/20 filter
Administration set (inner packaging):
1 single-use syringe with a capacity of 5 ml,
1 injection set,
2 alcohol swabs,
1 non-sterile plaster
Marketing authorization holder and manufacturer
CSL Behring GmbH
Emil-von-Behring-Strasse 76
35041 Marburg
Germany
This medicinal product is authorized in the Member States of the European Economic Area under the following names:
Berinert 1500 IE Pulver und Lösungsmittel zur Herstellung einer Injektions _____________________________ Austria
Berinert 1500 ___________________________ Belgium, Cyprus, Germany, Greece, Luxembourg, Poland
Berinert 1500, 500 IU Powder and solvent for solution for injection __________________ Bulgaria
Berinert 1500 IU ________________________ Czech Republic, Slovakia
Berinert _______________________________ Denmark, Italy, Portugal
Berinert 1500 IU, injektiokuiva-aine ja liuotin, liuosta varten ___________________ Finland
Berinert 1500 UI, poudre et solvant pour solution injectable ______________________ France
Berinert 1500 NE por és oldószer oldatos injekcióhoz ______________________ Hungary
Berinert 1500 UI, pulbere și solvent pentru soluție injectabilă _________________ Romania
Berinert 1500 i.e. prašek in vehikel za raztopino za injiciranje ___________________ Slovenia
Berinert 1500 UI Polvo para solución Inyectable _____________________________ Spain
Berinert 1500 IU powder and solvent for solution for injection __________________ United Kingdom
Date of approval of the leaflet: October 2021
Information intended for healthcare professionals only.
QUALITATIVE AND QUANTITATIVE COMPOSITION
The activity of human C1-esterase inhibitor is expressed in international units (IU), which refer to the current WHO standards for C1-esterase inhibitor products.
- Country of registration
- Active substance
- Prescription requiredYes
- ImporterCSL Behring GmbH
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to Berinert 1500Dosage form: Powder, 2000 IUActive substance: c1-inhibitor, plasma derivedPrescription requiredDosage form: Powder, 3000 IUActive substance: c1-inhibitor, plasma derivedPrescription requiredDosage form: Powder, 500 IU/mlActive substance: c1-inhibitor, plasma derivedPrescription required
Alternatives to Berinert 1500 in other countries
The best alternatives with the same active ingredient and therapeutic effect.
Alternative to Berinert 1500 in Ukraine
Alternative to Berinert 1500 in Spain
Online doctors for Berinert 1500
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for Berinert 1500 – subject to medical assessment and local rules.














