
CINRYZE 500 Units Powder and Solvent for Injectable Solution

How to use CINRYZE 500 Units Powder and Solvent for Injectable Solution
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the User
Cinryze 500 UI powder and solvent for solution for injection
human C1 esterase inhibitor
Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the pack
- What is Cinryze and what is it used for
- What you need to know before you use Cinryze
- How to use Cinryze
- Possible side effects
- Storage of Cinryze
- Contents of the pack and other information
1. What is Cinryze and what is it used for
Cinryze contains the human protein called "C1 esterase inhibitor" as the active substance.
The C1 esterase inhibitor is a protein that is produced naturally and is normally present in the blood. If the level of C1 esterase inhibitor in the blood is low or the C1 esterase inhibitor does not work properly, it can cause a swelling episode (called angioedema). The symptoms can include stomach pain and swelling of:
- hands and feet
- face, eyelids, lips or tongue
- larynx, which can make it difficult to breathe
- genitals
In adults and children, Cinryze can increase the amount of C1 esterase inhibitor in the blood and either prevent (before undergoing a medical or dental procedure) these swelling episodes from occurring or stop them once they have started.
In adults, adolescents and children (6 years of age and older), Cinryze can increase the amount of C1 esterase inhibitor in the blood and routinely prevent angioedema attacks.
2. What you need to know before you use Cinryze
Do not use Cinryze
- if you are allergic to human C1 esterase inhibitor or to any of the other ingredients of this medicine (listed in section 6). It is important that you inform your doctor if you think you have ever had an allergic reaction to any of the ingredients of Cinryze.
Warnings and precautions
- Before starting treatment with Cinryze, it is important that you inform your doctor if you have, or have had, blood clotting problems (thrombotic events). In this case, you will be closely monitored.
- If you start to experience skin rashes, chest tightness, wheezing or a fast heart beat after using Cinryze, inform your doctor immediately. See section 4.
- When medicines are made from human blood or plasma, certain measures are taken to prevent the transmission of infections to patients. These measures include the careful selection of plasma and blood donors to exclude people at risk of carrying infections, and the testing of each donation and plasma pool for signs of viruses/infections. The manufacturers of these products also include measures in the processing of blood or plasma that can inactivate and/or remove viruses. Despite these measures, when medicines made from human blood or plasma are administered, the possibility of transmitting an infection cannot be completely ruled out. This also applies to unknown or emerging viruses, or other types of infections.
- The measures taken are considered effective for enveloped viruses such as human immunodeficiency virus (HIV), hepatitis B virus and hepatitis C virus, and for non-enveloped viruses such as hepatitis A virus and parvovirus B19.
- Your doctor may recommend that you consider getting vaccinated against hepatitis A and B if you regularly or repeatedly receive human C1 esterase inhibitor products made from human plasma.
- In order to improve the traceability of biological medicines, the doctor or nurse must clearly record the name and batch number of the medicine administered.
Children
Cinryze should not be used in children under 6 years of age for routine prevention of angioedema attacks.
Using Cinryze with other medicines
Tell your doctor if you are using, have recently used or might use any other medicines.
Pregnancy and breastfeeding
If you are pregnant or breastfeeding, think you may be pregnant or are planning to have a baby, ask your doctor for advice before taking this medicine. The information on the safety of using Cinryze in pregnant and breastfeeding women is limited. Your doctor will inform you about the risks and benefits of using this medicine.
Driving and using machines
Cinryze has a minor influence on the ability to drive and use machines.
Cinryze contains sodium
This medicine contains 11.5 mg of sodium (a major component of cooking/table salt) in each vial. This is equivalent to 0.5% of the maximum daily intake of sodium recommended for an adult.
3. How to use Cinryze
Your treatment will be started and controlled under the supervision of a doctor with experience in the care of patients with hereditary angioedema (HAE).
Your doctor or nurse may prepare and administer the Cinryze injection to you. If your doctor decides that you can self-administer the medicine, your doctor or nurse will teach you or a family member how to prepare and inject Cinryze. Your doctor will review the preparation and administration process with you or with a family member or caregiver periodically.
The recommended dose of Cinryze for adults, adolescents, children, elderly or patients with liver or kidney problems is as follows:
Use in adults and adolescents (12 years of age and older)
Treatment of swelling episodes
- A dose of 1,000 UI (two vials) of Cinryze should be injected at the first sign of a swelling episode.
- A second injection of 1,000 UI may be administered if symptoms do not improve after 60 minutes.
- If you experience a severe attack, especially laryngeal swelling, or if treatment is delayed, a second dose of 1,000 UI may be administered before 60 minutes have passed since the first dose, depending on your clinical response.
- Cinryze will be administered by intravenous injection (into a vein).
Routine prevention of swelling episodes
- A dose of 1,000 UI (two vials) of Cinryze should be injected every 3 or 4 days for routine prevention of swelling episodes.
- Your doctor may adjust the administration interval based on your response to Cinryze.
- Cinryze will be administered by intravenous injection (into a vein).
Preoperative prevention of swelling episodes
- A dose of 1,000 UI (two vials) of Cinryze should be injected up to 24 hours before a medical, dental or surgical procedure.
- Cinryze will be administered by intravenous injection (into a vein).
Use in children
Treatment of angioedema attacks | Preoperative prevention of angioedema attacks | Routine prevention of angioedema attacks |
2 to 11 years, > 25 kg: A dose of 1,000 UI (two vials) of Cinryze should be injected at the first sign of a swelling episode. A second injection of 1,000 UI may be administered if symptoms do not improve after 60 minutes. 2 to 11 years, 10-25 kg: A dose of 500 UI (one vial) of Cinryze should be injected at the first sign of a swelling episode. A second injection of 500 UI may be administered if symptoms do not improve after 60 minutes. | 2 to 11 years, > 25 kg: A dose of 1,000 UI (two vials) of Cinryze should be injected up to 24 hours before a medical, dental or surgical procedure. 2 to 11 years, 10-25 kg: A dose of 500 UI (one vial) of Cinryze should be injected up to 24 hours before a medical, dental or surgical procedure. | 6 to 11 years: A dose of 500 UI (one vial) of Cinryze should be injected every 3 or 4 days for routine prevention of swelling episodes. Your doctor may adjust the administration interval based on your response to Cinryze. |
Reconstitution and administration
Normally, it will be your doctor or nurse who will administer the Cinryze injection to you in a vein (intravenously). You or your caregiver may also be responsible for administering Cinryze by injection, but only after receiving proper training. If you administer Cinryze yourself, always follow your doctor's instructions exactly. If in doubt, ask your doctor. If your doctor decides that you can receive home treatment, they will give you detailed instructions. You will be asked to complete a diary to document each treatment administered at home and to bring it to each of your doctor's appointments. You/your caregiver will undergo periodic reviews of the injection technique to ensure that you are handling the medicine properly.
If you have any further questions on the use of this medicine, ask your doctor or pharmacist.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
This can include allergic reactions.
Tell your doctor immediatelyif you experience any of the following symptoms after using this medicine. Although rare, the symptoms can be severe.
Sudden wheezing, difficulty breathing, swelling of the eyelids, face or lips, skin rash or itching (especially if it affects the whole body).
Very common side effects (may affect more than 1 in 10 people): headache, nausea.
Common side effects (may affect up to 1 in 10 people): hypersensitivity, dizziness, vomiting, skin rash or itching or redness, injection site reaction or pain, fever.
Uncommon side effects (may affect up to 1 in 100 people): high blood sugar levels, blood clots, vein pain, flushing, cough, stomach pain, diarrhea, skin peeling, joint swelling and pain, muscle pain and chest discomfort.
It is expected that the side effects in children and adolescents will be similar to those in adults.
Reporting of side effects
If you experience any side effects, talk to your doctor or nurse, even if they are not listed in this leaflet. You can also report side effects directly via the national reporting system listed in Appendix V. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of Cinryze
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date which is stated on the carton and on the vials after "EXP".
Store below 25°C. Do not freeze. Store in the original package to protect from light.
Once reconstituted, the Cinryze solution should be used immediately.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. This will help protect the environment.
6. Container Contents and Additional Information
Composition ofCinryze
The active ingredient is human C1 esterase inhibitor, manufactured from human plasma donors. Each vial of powder contains 500 UI of human C1 esterase inhibitor. After reconstitution, one vial contains 500 UI of human C1 esterase inhibitor per 5 ml, which is equivalent to a concentration of 100 UI/ml. Two vials of reconstituted Cinryze contain 1,000 UI of human C1 esterase inhibitor per 10 ml, which is equivalent to a concentration of 100 UI/ml.
The total protein content of the reconstituted solution is 15 ± 5 mg/ml.
One international unit (UI) is equivalent to the amount of C1 esterase inhibitor present in 1 ml of normal human plasma.
The other components are sodium chloride, sucrose, sodium citrate, L-valine, L-alanine, and L-threonine (see section 2).
Solvent: water for injectable preparations.
Appearance of the Product and Container Contents
Powder and solvent for injectable solution.
Cinryze is a white powder that comes in a vial.
After dissolution in water for injectable preparations, the solution is clear and colorless to slightly blue.
Each container contains:
2 vials of Cinryze 500 UI powder for injectable solution
2 vials of water for injectable preparations (5 ml each)
2 filter transfer devices
2 disposable 10 ml syringes
2 venipuncture devices
2 protectors
Use only a silicone-free syringe (provided in the container) for medication administration.
Marketing Authorization Holder and Manufacturer
Marketing Authorization Holder
Takeda Manufacturing Austria AG
Industriestrasse 67
1221 Vienna
Austria
Manufacturer
Takeda Manufacturing Austria AG
Industriestrasse 67
1221 Vienna
Austria
Shire International Licensing B.V.
Mercuriusplein 11
2132 HA Hoofddorp
Netherlands
You can request more information about this medication by contacting the local representative of the marketing authorization holder:
Belgium/Belgique/Belgien Takeda Belgium NV Tel: +32 2 464 06 11 | Lithuania Takeda, UAB Tel: +370 521 09 070 |
| Luxembourg/Luxemburg Takeda Belgium NV Tel: +32 2 464 06 11 |
Czech Republic Takeda Pharmaceuticals Czech Republic s.r.o. Tel: +420 234 722 722 | Hungary Takeda Pharma Kft. Tel: +36 1 270 7030 |
Denmark Takeda Pharma A/S Tel: +45 46 77 10 10 | Malta Takeda HELLAS S.A. Tel: +30 210 6387800 |
Germany Takeda GmbH Tel: +49 (0)800 825 3325 | Netherlands Takeda Nederland B.V. Tel: +31 20 203 5492 |
Estonia Takeda Pharma OÜ Tel: +372 6177 669 | Norway Takeda AS Tel: +47 800 800 30 |
Greece Takeda ΕΛΛΑΣ Α.Ε. Tel: +30 210 6387800 | Austria Takeda Pharma Ges.m.b.H. Tel: +43 (0) 800-20 80 50 |
Spain Takeda Farmacéutica España S.A. Tel: +34 917 90 42 22 | Poland Takeda Pharma Sp. z o.o. Tel: +48223062447 |
France Takeda France SAS Tel: + 33 1 40 67 33 00 | Portugal Takeda Farmacêuticos Portugal, Lda. Tel: + 351 21 120 1457 |
Croatia Takeda Pharmaceuticals Croatia d.o.o. Tel: +385 1 377 88 96 | Romania Takeda Pharmaceuticals SRL Tel: +40 21 335 03 91 |
Ireland Takeda Products Ireland Ltd Tel: 1800 937 970 | Slovenia Takeda Pharmaceuticals farmacevtska družba d.o.o. Tel: + 386 (0) 59 082 480 |
Iceland Vistor ehf. Tel: +354 535 7000 | Slovakia Takeda Pharmaceuticals Slovakia s.r.o. Tel: +421 (2) 20 602 600 |
Italy Takeda Italia S.p.A. Tel: +39 06 502601 | Finland Takeda Oy Tel: 0800 774 051 |
Cyprus Takeda ΕΛΛΑΣ Α.Ε. Tel: +30 210 6387800 | Sweden Takeda Pharma AB Tel: 020 795 079 |
Latvia Takeda Latvia SIA Tel: +371 67840082 |
Date of the Last Revision of this Prospectus:
Other Sources of Information
Detailed information about this medication is available on the European Medicines Agency website: https://www.ema.europa.eu. There are also links to other websites about rare diseases and orphan medicines.
____________________________________________________________________________
This information is intended only for healthcare professionals:
Reconstitution and Administration of Cinryze
Reconstitution, product administration, and handling of the administration equipment and needles should be performed with caution.
Use the filter transfer device provided with Cinryze or a commercially available double-pointed needle.
Use only a silicone-free syringe (provided in the container) for medication administration.
Preparation and Handling
Cinryze is indicated for intravenous administration (in the vein) after reconstitution with water for injectable preparations.
The Cinryze vial is for single use only.
Reconstitution
For a 500 UI dose: 1 vial of powder, 1 vial of solvent, 1 filter transfer device, 1 disposable 10 ml syringe, 1 venipuncture device, and 1 protector are needed. Save the remaining vial and administration equipment for the next dose.
For a 1,000 UI dose: 2 vials of powder, 2 vials of solvent, 2 filter transfer devices, 1 disposable 10 ml syringe, 1 venipuncture device, and 1 protector are needed.
Each vial of product should be reconstituted with 5 ml of water for injectable preparations.
A reconstituted Cinryze vial corresponds to a 500 UI dose. Therefore, reconstitute only one Cinryze vial for a 500 UI dose.
Two reconstituted Cinryze vials correspond to a 1,000 UI dose. Therefore, for a 1,000 UI dose, combine two vials.
- Work on the provided protector and wash your hands before performing the following procedures.
- Use an aseptic technique during the reconstitution procedure.
- Ensure that the powder vial and solvent vial are at room temperature (15°C – 25°C).
- Remove the label from the powder vial by peeling off the purple strip indicating the arrow.
- Remove the seals from the powder and solvent vials.
- Clean the stoppers with a disinfectant wipe and let them dry before use.
- Remove the seal from the top of the transfer device container. Do not remove the device from the container.
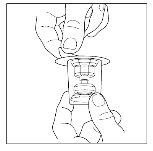
- Note: The transfer device must be attached to the solvent vial before being attached to the powder vial, so that the vacuum in the powder vial is not lost. Place the solvent vial on a flat surface and insert the blue end of the transfer device into the solvent vial, pushing it in until the point penetrates the center of the vial stopper and the device clicks into place. The transfer device should be vertical before penetrating the stopper.
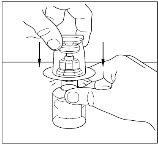
- Remove the seal from the transfer device and discard it. Be careful not to touch the exposed end of the transfer device.
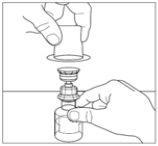
- Place the powder vial on a flat surface. Invert the transfer device and the solvent vial containing the water for injectable preparations, and insert the transparent end of the transfer device into the powder vial, pushing it in until the point penetrates the rubber stopper and the transfer device clicks into place. The transfer device should be vertical before penetrating the stopper of the powder vial. The vacuum in the powder vial will extract the solvent. If the vial does not have a vacuum, do not use the product.
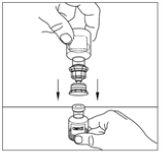
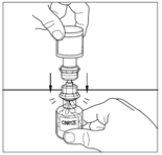
- Gently move the powder vial until it is well dissolved. Do not shake the powder vial. Ensure that all the powder is well dissolved.
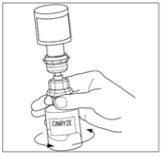
- Disconnect the solvent vial by turning it to the left. Do not remove the transparent end of the transfer device from the powder vial.
A reconstituted Cinryze vial contains 500 UI of human C1 esterase inhibitor in 5 ml, resulting in a concentration of 100 UI/ml. Proceed to the administration process if patients receive a 500 UI dose.
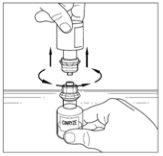
Two Cinryze powder vials should be reconstituted to make a dose (1,000 UI/10 ml). Therefore, repeat steps 1 to 12 above, using a new container with a transfer device to reconstitute the second powder vial. Do not reuse the same transfer device. Once the two vials have been reconstituted, proceed to the administration process for a 1,000 UI dose.
Administration Process for a 500 UI Dose
- Use an aseptic technique during the administration procedure.
- After reconstitution, the Cinryze solutions are clear and colorless to slightly blue. Do not use the product if the solutions are cloudy or discolored.
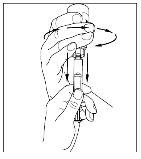
- With a sterile and disposable 10 ml syringe, pull the plunger to draw in approximately 5 ml of air into the syringe.
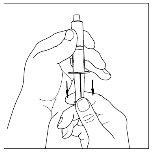
- Attach the syringe to the top of the transparent end of the transfer device by turning it to the right.
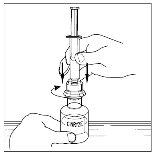
- Invert the vial carefully and inject air into the solution, then slowly extract the reconstituted Cinryze solution into the syringe.
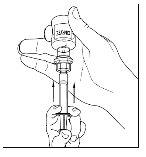
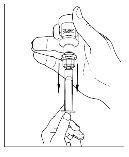
- Disconnect the syringe from the vial by turning it to the left and releasing it from the transparent end of the transfer device.
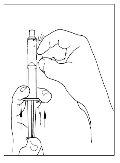
- Before administration, inspect the reconstituted Cinryze solution for particles. Do not use the medication if you observe particles.
- Attach the venipuncture device to the syringe containing the Cinryze solution and administer the injection intravenously (in the vein) to the patient. Administer 500 UI (reconstituted in 5 ml of water for injectable preparations) of Cinryze by intravenous injection at a rate of 1 ml per minute for 5 minutes.
Administration Process for a 1,000 UI Dose
- Use an aseptic technique during the administration procedure.
- After reconstitution, the Cinryze solutions are clear and colorless to slightly blue. Do not use the product if the solutions are cloudy or discolored.
- With a sterile and disposable 10 ml syringe, pull the plunger to draw in approximately 5 ml of air into the syringe.
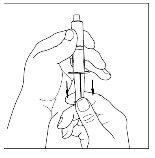
- Attach the syringe to the top of the transparent end of the transfer device by turning it to the right.
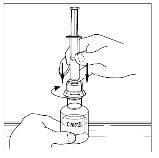
- Invert the vial carefully and inject air into the solution, then slowly extract the reconstituted Cinryze solution into the syringe.
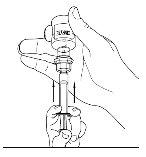
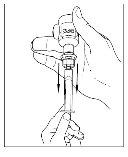
- Disconnect the syringe from the vial by turning it to the left and releasing it from the transparent end of the transfer device.
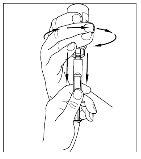
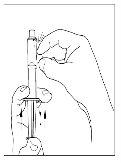
- Using the same syringe, repeat steps 3 to 6 with the second reconstituted Cinryze vial to make a complete dose of 10 ml.
- Before administration, inspect the reconstituted Cinryze solution for particles. Do not use the medication if you observe particles.
- Attach the venipuncture device to the syringe containing the Cinryze solution and administer the injection intravenously (in the vein) to the patient. Administer 1,000 UI (reconstituted in 10 ml of water for injectable preparations) of Cinryze by intravenous injection at a rate of 1 ml per minute for 10 minutes.
The disposal of unused medication and all materials that have come into contact with it will be carried out in accordance with local regulations.
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to CINRYZE 500 Units Powder and Solvent for Injectable SolutionDosage form: INJECTABLE, 1500 IUActive substance: c1-inhibitor, plasma derivedManufacturer: Csl Behring GmbhPrescription requiredDosage form: INJECTABLE, 2000 IUActive substance: c1-inhibitor, plasma derivedManufacturer: Csl Behring GmbhPrescription requiredDosage form: INJECTABLE, 3000 IUActive substance: c1-inhibitor, plasma derivedManufacturer: Csl Behring GmbhPrescription required
Online doctors for CINRYZE 500 Units Powder and Solvent for Injectable Solution
Discuss questions about CINRYZE 500 Units Powder and Solvent for Injectable Solution, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions
















