
Aurodisc
Ask a doctor about a prescription for Aurodisc

How to use Aurodisc
Leaflet accompanying the packaging: patient information
Aurodisc, (50 micrograms + 500 micrograms)/dose, inhalation powder, divided
Salmeterol + Fluticasone propionate
You should carefully read the contents of the leaflet before using the medicine, as it contains important information for the patient.
- You should keep this leaflet, so that you can read it again if you need to.
- In case of any doubts, you should consult a doctor, pharmacist, or nurse.
- This medicine has been prescribed to a specific person. Do not pass it on to others. The medicine may harm another person, even if their symptoms are the same.
- If the patient experiences any side effects, including any side effects not listed in this leaflet, they should tell their doctor, pharmacist, or nurse. See section 4.
Table of contents of the leaflet
- 1. What is Aurodisc and what is it used for
- 2. Important information before using Aurodisc
- 3. How to use Aurodisc
- 4. Possible side effects
- 5. How to store Aurodisc
- 6. Contents of the packaging and other information
1. What is Aurodisc and what is it used for
Aurodisc contains two active substances: salmeterol and fluticasone propionate.
- Salmeterol is a long-acting bronchodilator. Bronchodilators help maintain the patency of the airways. This facilitates the flow of air to and from the lungs. The effect lasts for at least 12 hours.
- Fluticasone propionate is a corticosteroid that reduces swelling and irritation of the lungs.
Aurodisc is used in the treatment of adults and adolescents aged 12 years and older.
The doctor has prescribed this medicine to the patient to prevent breathing disorders that occur in:
- asthma, or
- chronic obstructive pulmonary disease (COPD). Salmeterol and fluticasone propionate, at a dose of 50 micrograms + 500 micrograms, reduce the number of exacerbations of COPD symptoms.
To ensure proper control of asthma or COPD, Aurodisc must be used every day as recommended by the doctor.
Aurodisc prevents shortness of breath and wheezing in the airways. However, it should not be used to control sudden attacks of shortness of breath or wheezing in the airways.
If such an attack occurs, it is necessary to use a rapidly acting bronchodilator, such as salbutamol, immediately. The patient should always carry such a medicine with them.
2. Important information before using Aurodisc
When not to use Aurodisc
- If the patient is allergic to salmeterol, fluticasone propionate, or lactose monohydrate,
a helper substance in the medicine.
Warnings and precautions
Before starting to use Aurodisc, the patient should talk to their doctor, pharmacist, or nurse if they have:
- heart disease, including irregular or rapid heartbeat,
- hyperthyroidism,
- high blood pressure,
- diabetes (Aurodisc may increase blood glucose levels),
- low potassium levels in the blood,
- tuberculosis currently being treated or having been treated, or other lung infections.
If the patient experiences blurred vision or other vision disturbances, they should contact their doctor.
Children
This medicine should not be used in children under the age of 12.
Aurodisc and other medicines
The patient should tell their doctor about all medicines they are currently taking or have recently taken, as well as any medicines they plan to take. Aurodisc should not be used with certain medicines.
Before starting to use Aurodisc, the patient should inform their doctor about taking the following medicines:
- Beta-adrenergic blockers (e.g., atenolol, propranolol, and sotalol). Beta-adrenergic blockers are most often used to treat high blood pressure or other heart diseases, such as angina pectoris.
- Medicines used to treat infections (e.g., ketoconazole, itraconazole, and erythromycin), including some medicines used to treat HIV (e.g., ritonavir, cobicistat-containing medicines). Some of these medicines may increase the levels of fluticasone propionate or salmeterol in the body. This may increase the risk of side effects of Aurodisc, including irregular heartbeat, or may worsen existing side effects. The doctor may want to closely monitor the patient's condition when taking such medicines.
- Corticosteroids (orally or by injection). If the patient has recently taken such medicines, it may increase the risk of adrenal insufficiency caused by Aurodisc.
- Diuretics used to treat high blood pressure.
- Other bronchodilators (such as salbutamol).
- Medicines containing xanthine derivatives, such as aminophylline and theophylline, often used to treat asthma.
Pregnancy and breastfeeding
If the patient is pregnant or breastfeeding, thinks they may be pregnant, or plans to have a child, they should consult their doctor before using this medicine.
Driving and using machines
It is unlikely that Aurodisc will affect the ability to drive and use machines.
Aurodisc contains lactose
Aurodisc contains about 13 milligrams of lactose monohydrate per dose. The amount of lactose in this medicine usually does not cause problems for people with lactose intolerance. The helper substance lactose contains small amounts of milk proteins, which may cause allergic reactions.
3. How to use Aurodisc
This medicine should always be used as recommended by the doctor. In case of doubts, the patient should consult their doctor or pharmacist.
- Aurodisc should be used every day, unless the doctor recommends otherwise. Do not take a higher dose than recommended. In case of doubts, the patient should consult their doctor or pharmacist.
- Do not stop using Aurodisc or reduce the dose without consulting the doctor.
- Aurodisc should be inhaled into the lungs through the mouth.
Recommended dose
Asthma
Adults and adolescents aged 12 years and older
- Aurodisc (50 micrograms + 500 micrograms)/dose: one inhalation twice a day.
Adults with chronic obstructive pulmonary disease (COPD)
- Aurodisc (50 micrograms + 500 micrograms)/dose: one inhalation twice a day.
If the symptoms of asthma are well-controlled while using Aurodisc twice a day, the doctor may recommend reducing the frequency of use to once a day. The dose can be given:
- once a day, in the evening, if the patient's symptoms occur at night,
- once a day, in the morning, if the patient's symptoms occur during the day. It is very important that the doctor instructs the patient on how many inhalations and how often to use.
If the patient is using Aurodisc for asthma, the doctor will regularly check the symptoms.
In case of worsening asthma symptoms or breathing difficulties, the patient should immediately contact their doctor.
There may be an increase in wheezing, more frequent chest tightness, or the need to use a higher dose of a rapidly acting inhalation medicine to facilitate breathing. In any of these situations, the patient should continue using Aurodisc, but not increase the number of inhalations. The symptoms of the disease may worsen, and the patient's condition may deteriorate. The patient should contact their doctor, as they may need additional treatment.
Instructions for use
- Aurodisc may differ from inhalers used by the patient in the past, so it is very important to use it correctly. The doctor, nurse, or pharmacist should train the patient in using the inhaler. This training is important to ensure that the patient receives the required dose. If the patient has not received this training, they should ask their doctor, nurse, or pharmacist to show them how to use the inhaler correctly, especially before the first use.They should also periodically check that the patient is using the inhalation device correctly. Using Aurodisc not in accordance with the doctor's recommendation may cause the medicine not to produce the expected improvement in asthma or COPD.
- The inhalation device contains blisters with salmeterol and fluticasone propionate in powder form.
- The inhalation device is equipped with a dose counter, which indicates how many doses of the medicine are left in the inhalation device. The counter indicates the dose number down to 0. The numbers from 5 to 0 appear in red to warn that there are only a few doses of the medicine left in the inhalation device. If the counter shows 0, it means that the inhalation device is empty.
Using the inhalation device
- 1. To open the inhalation device, the patient should hold the device in one hand in a horizontal position. They should press the red button with their thumb (see Fig. 1) and turn the purple (for 50/500 micrograms) mouthpiece cover away from them with the thumb of the other hand until it clicks (see Fig. 2). This will open a small hole in the mouthpiece and place a dose of the medicine in the mouthpiece.
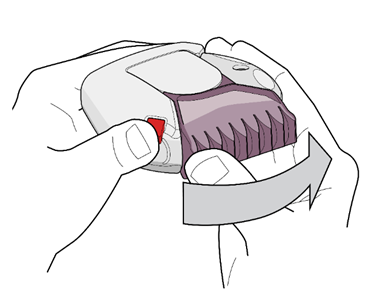
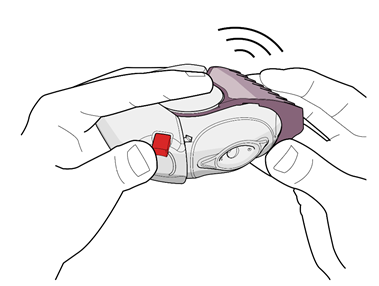
Fig. 1
Fig. 2
Note: Each time the mouthpiece cover is opened after the "click", a blister opens inside, and the next dose of powder is ready for inhalation. Therefore, the mouthpiece cover should not be opened unless the medicine is to be taken, as the next blister will open, and the medicine will be wasted.
- 2. The inhalation device should be held at a distance from the mouth and a slow, deep exhalation should be performed. Do not exhale into the inhalation device.
- 3. The mouthpiece should be placed in the mouth (see Fig. 3). A deep inhalation should be performed from the inhalation device through the mouth, not through the nose. The inhalation device should be removed from the mouth. The breath should be held for about 10 seconds or as long as it is comfortable. A slow exhalation should be performed.
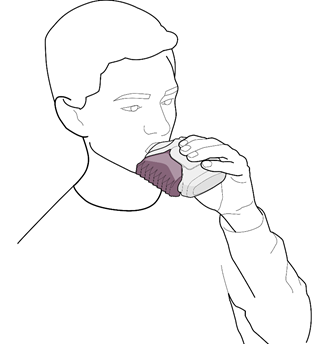
Fig. 3
The inhaler delivers a dose of the medicine in the form of a very fine powder. The patient may, but does not have to, feel the powder. Do not take an additional dose from the inhaler if the patient does not feel the taste of the medicine.
- 4. Close the inhaler to keep it clean by turning the purple (for 50/500 micrograms) mouthpiece cover back as far as possible. The patient will hear a "click" (see Fig. 4). The mouthpiece cover has now been restored to its original position. The inhaler is now ready for use at the next scheduled dose.
- 5. After inhaling the medicine, the patient should rinse their mouth with water and spit it out and (or) brush their teeth. This may help prevent hoarseness and thrush.
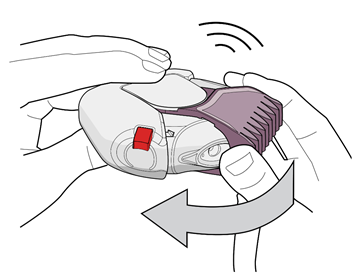
Fig. 4
Cleaning the inhaler
Store the inhaler dry and clean.
If necessary, the mouthpiece of the inhalation device can be wiped with a dry cloth or tissue.
Using a higher dose of Aurodisc than recommended
It is important to use the inhalation device as recommended. If a higher dose than recommended is used by mistake, the patient should tell their doctor or pharmacist. The following may occur: faster than normal heartbeat, tremors, dizziness, headache, weakness, and joint pain.
If higher doses are used for a long time, the patient should contact their doctor or pharmacist for advice, as high doses of Aurodisc may cause a decrease in the production of steroid hormones by the adrenal glands.
Missing a dose of Aurodisc
Do not take a double dose of the medicine to make up for a missed dose. The next dose should be taken at the scheduled time.
Stopping the use of Aurodisc
It is very important to take Aurodisc every day as recommended by the doctor. Do not stop taking the medicine unless the doctor recommends it. Do not suddenly stop taking Aurodisc or reduce the dose, as the symptoms of the disease may worsen.
Additionally, sudden stopping or reducing the dose of Aurodisc may (very rarely) cause adrenal insufficiency, which can sometimes cause side effects.
These side effects may include:
- abdominal pain,
- fatigue and loss of appetite, nausea,
- vomiting and diarrhea,
- weight loss,
- headache and drowsiness,
- low blood sugar,
- low blood pressure and seizures.
When the body is under stress, such as from fever, injury (such as a car accident), infection, or surgery, adrenal insufficiency may worsen, and any of the above side effects may occur.
If the patient experiences any of these side effects, they should tell their doctor or pharmacist. To prevent these symptoms, the doctor may prescribe additional corticosteroids in the form of tablets (e.g., prednisolone).
In case of any further doubts about using this medicine, the patient should consult their doctor, pharmacist, or nurse.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
To minimize the risk of side effects, the doctor will recommend the smallest dose of this medicine that will control asthma or COPD.
Allergic reactions: The patient may experience sudden breathing difficulties immediately after using Aurodisc.
The patient may experience an increase in wheezing, coughing, or shortness of breath, as well as itching, rash (hives), and swelling (usually of the face, lips, tongue, or throat). The patient may also experience a feeling of a very fast heartbeat, weakness, and dizziness (which may lead to falling or loss of consciousness). In case of any of these symptoms, including if they occur suddenly after using Aurodisc, the patient should stop using Aurodisc and contact their doctor immediately.Allergic reactions to Aurodisc are uncommon (may occur less often than in 1 in 100 people).
Pneumonia (lung infection) in patients with chronic obstructive pulmonary disease (COPD) (common side effect)
The patient should tell their doctorif they experience any of the following symptoms while using Aurodisc - these may be symptoms of a lung infection:
- Fever or chills.
- Increased production of mucus, change in the color of mucus.
- Worsening cough or increased breathing difficulties.
Other side effects:
Very common side effects (may occur more often than in 1 in 10 people)
- Headache - this side effect usually decreases over time with continued treatment.
- Increased frequency of colds in patients with chronic obstructive pulmonary disease (COPD) has been reported.
Common side effects (may occur less often than in 1 in 10 people)
- Thrush (painful, creamy-white patches) in the mouth and throat, as well as sore tongue, hoarseness, and throat irritation. Rinsing the mouth with water and spitting it out and (or) brushing the teeth after each inhalation may be helpful. The doctor may recommend an antifungal medicine to treat thrush.
- Pain, swelling of the joints, and muscle pain.
- Muscle cramps.
The following side effects have been reported in patients with chronic obstructive pulmonary disease (COPD):
- Easier bruising and fractures.
- Sinusitis (feeling of tension and fullness in the nose, cheeks, and behind the eyes, sometimes with a pulsating headache).
- Decreased potassium levels in the blood (the patient may experience irregular heartbeat, muscle weakness, cramps).
Uncommon side effects (may occur less often than in 1 in 100 people)
- Increased blood sugar (glucose) levels (hyperglycemia). In patients with diabetes, it may be necessary to monitor blood sugar levels more frequently and adjust the dose of antidiabetic medicines being taken.
- Cataract (clouding of the lens of the eye).
- Very fast heartbeat (tachycardia).
- Feeling of trembling and fast or irregular heartbeat (palpitations) - these symptoms usually are not serious and decrease over time with continued treatment.
- Chest pain.
- Restlessness (this side effect occurs mainly in children).
- Sleep disturbances.
- Allergic skin rash.
Rare side effects (may occur less often than in 1 in 1000 people)
- Breathing difficulties or wheezing worsening immediately after taking Aurodisc. In case of such symptoms, the patient should stop using Aurodisc, use a rapidly acting inhalation medicine to facilitate breathing, and contact their doctor immediately.
- Aurodisc may disrupt the normal production of steroid hormones by the body, especially when taking high doses of the medicine for a long time. These symptoms include:
- slowed growth in children and adolescents,
- decreased bone mass,
- glaucoma,
- weight gain,
- rounding (moon-shaped face) (Cushing's syndrome). The doctor will regularly check if the patient is experiencing such side effects and ensure that the patient is taking the smallest dose of this medicine that will control asthma.
- Changes in behavior, such as excessive excitement and irritability (these symptoms occur mainly in children).
- Irregular, irregular heartbeat or extra beats (heart rhythm disturbances). The patient should inform their doctor about this, but should not stop using Aurodisc unless the doctor recommends it.
- Fungal infection of the esophagus, which can cause difficulty swallowing.
Side effects with unknown frequency (frequency cannot be estimated from the available data)
- Depression or aggression (the occurrence of these side effects is more likely in children).
- Blurred vision.
Reporting side effects
If the patient experiences any side effects, including any side effects not listed in this leaflet, they should tell their doctor or pharmacist. Side effects can be reported directly to the Department of Monitoring of Adverse Reactions to Medicinal Products of the Office for Registration of Medicinal Products, Medical Devices, and Biocidal Products:
Aleje Jerozolimskie 181C, 02-222 Warsaw, tel.: 22 49-21-301, fax: 22 49-21-309, website:
https://smz.ezdrowie.gov.pl
Side effects can also be reported to the marketing authorization holder.
By reporting side effects, more information can be collected on the safety of this medicine.
5. How to store Aurodisc
- •The medicine should be stored out of sight and reach of children.
- Do not use this medicine after the expiry date stated on the carton and label of the inhaler after: EXP. The expiry date (EXP) means the last day of the month stated.
- Do not store above 30°C.
- Medicines should not be disposed of via wastewater or household waste. The patient should ask their pharmacist how to dispose of medicines that are no longer needed. This will help protect the environment.
6. Contents of the packaging and other information
What Aurodisc contains
The active substances of the medicine are salmeterol and fluticasone propionate.
What Aurodisc looks like and contents of the pack
- Aurodisc contains a strip of foil with blisters filled with white or off-white powder. The foil protects the inhalation powder from environmental conditions.
- Each dose is divided.
- The white plastic device with a purple (for 50/500 micrograms) mouthpiece cover is packaged in cardboard boxes, which contain: 1, 2, 3, or 10 inhalers, each containing 60 doses of powder.
Not all pack sizes may be marketed.
Marketing authorization holder
Aurovitas Pharma Polska Sp. z o.o.
ul. Sokratesa 13D lokal 27
01-909 Warsaw
Manufacturer/Importer
Oy Medfiles Ltd.
Volttikatu 5, Volttikatu 8
70700 Kuopio
Finland
APL Swift Services (Malta) Ltd
HF26, Hal Far Industrial Estate, Hal Far,
3000 Birzebbugia, BBG
Malta
Special Product’s Line S.p.A.
Via Fratta Rotonda Vado Largo, 1
- 03012 – Anagni (FR) Italy
This medicinal product is authorized in the Member States of the European Economic Area under the following names:
Belgium
Aurocombo 50 microgram/500 microgram/dose, inhalation powder, pre-dispensed
Aurocombo 50 micrograms/500 micrograms/dose, powder for inhalation in a single-dose container
Aurocombo 50 Mikrogramm/500 Mikrogramm single-dose powder for inhalation
Czech Republic
Aurasth
France
PROPIONATE DE FLUTICASONE/SALMETEROL ARROW 500 micrograms/50 micrograms/dose, powder for inhalation in a single-dose container
Germany
Salmeterol/Fluticasonpropionat Zentiva Airmaster 50 Mikrogramm/500 Mikrogramm single-dose powder for inhalation
Hungary
Sirmin 50 mikrogramm/500 mikrogramm/dose, single-dose inhalation powder
Italy
BISKUS
Netherlands
Serair 50 microgram/500 microgram inhalation powder, pre-dispensed
Poland
Aurodisc
Romania
Aurospir 50 micrograms/500 micrograms single-dose inhalation powder
Sweden
Campona Airmaster
United Kingdom
Campona Airmaster
Date of last revision of the leaflet: 10/2021
- Country of registration
- Active substance
- Prescription requiredYes
- ImporterAPL Swift Services (Malta) Ltd. Oy Medfiles Ltd. SPECIAL PRODUCTS LINE S.p.A.
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to AurodiscDosage form: Powder, 50 mcg + 250 mcgActive substance: salmeterol and fluticasoneManufacturer: Aeropharm GmbHPrescription requiredDosage form: Powder, 50 mcg + 500 mcgActive substance: salmeterol and fluticasoneManufacturer: Aeropharm GmbHPrescription requiredDosage form: Powder, (50 micrograms + 500 micrograms)/doseActive substance: salmeterol and fluticasonePrescription required
Alternatives to Aurodisc in other countries
The best alternatives with the same active ingredient and therapeutic effect.
Alternative to Aurodisc in Іспанія
Alternative to Aurodisc in Україна
Online doctors for Aurodisc
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for Aurodisc – subject to medical assessment and local rules.














