
ASERIFLU 50/500 micrograms inhalation powder (single dose)

How to use ASERIFLU 50/500 micrograms inhalation powder (single dose)
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Leaflet: information for the user
Aseriflu 50 micrograms/250 micrograms/inhalation powder for inhalation (single-dose)
Aseriflu 50 micrograms/500 micrograms/inhalation powder for inhalation (single-dose)
salmeterol/fluticasone propionate
Read this leaflet carefully before you start using this medicine because it contains important information for you.
- Keep this leaflet, as you may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their symptoms are the same as yours.
- If you experience any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the pack
- What Aseriflu is and what it is used for
- What you need to know before you use Aseriflu
- How to use Aseriflu
- Possible side effects
- Storing Aseriflu
- Contents of the pack and other information
1. What Aseriflu is and what it is used for
Aseriflu contains two active substances, salmeterol and fluticasone propionate:
- Salmeterol is a long-acting bronchodilator. Bronchodilators help to keep the airways open in the lungs, making it easier to breathe in and out. The effects last for at least 12 hours.
- Fluticasone propionate is a corticosteroid that reduces inflammation and irritation in the lungs.
Your doctor has prescribed this medicine to help prevent breathing problems such as:
- Asthma.
- Chronic Obstructive Pulmonary Disease (COPD). Salmeterol and fluticasone propionate, in a dose of 50/500 micrograms, reduce the number of exacerbations of COPD symptoms.
You should use this medicine every day as your doctor has recommended. This will ensure that the medication works correctly to control your asthma or COPD.
Aseriflu helps to prevent shortness of breath and wheezing. However, Aseriflu should not be used to relieve a sudden attack of shortness of breath or wheezing. In such cases, you should use your "rescue" medication of rapid action, such as salbutamol. You should always carry your rapid-acting rescue medication with you.
2. What you need to know before you use Aseriflu
Do not use Aseriflu
- if you are allergic to salmeterol, fluticasone propionate, or the other component, lactose monohydrate.
Warnings and precautions
Talk to your doctor before you start using this medicine if you have:
- Heart problems, including a fast or irregular heartbeat.
- Overactive thyroid gland.
- High blood pressure.
- Diabetes mellitus (this medicine may increase blood sugar levels).
- Low levels of potassium in the blood.
- Tuberculosis (TB) now or in the past, or other lung infections.
If you experience blurred vision or other visual disturbances, contact your doctor.
Children and adolescents
Aseriflu should not be used in children under 12 years of age.
Other medicines and Aseriflu
Tell your doctor or pharmacist if you are using, have recently used, or might use any other medicines, including those for asthma or those bought without a prescription. The reason is that, in some cases, Aseriflu should not be taken with other medicines.
Tell your doctor if you are taking any of the following medicines before you start using Aseriflu:
- β-blockers (such as atenolol, propranolol, and sotalol). β-blockers are mainly used to treat high blood pressure or other heart conditions.
- Medicines for treating infections (such as ketoconazole, itraconazole, and erythromycin), including some medicines for HIV (such as ritonavir, medicines containing cobicistat). Some of these medicines may increase the amount of fluticasone propionate or salmeterol in your body. This may increase your risk of experiencing side effects with Aseriflu, including irregular heartbeats, or may worsen side effects, so your doctor will monitor you closely if you are taking these medicines.
- Corticosteroids (oral or injectable). If you have taken these medicines recently, you may be at increased risk of this medicine affecting your adrenal glands.
- Diuretics, also known as water pills, used to treat high blood pressure.
- Other bronchodilators (such as salbutamol).
- Medicines containing xanthines. These are often used to treat asthma.
Pregnancy and breastfeeding
If you are pregnant or breastfeeding, think you may be pregnant, or plan to become pregnant, consult your doctor or pharmacist before using this medicine.
Driving and using machines
Aseriflu is unlikely to affect your ability to drive or use machines unless you experience side effects such as blurred vision.
Aseriflu contains lactose
This medicine contains up to 12 mg of lactose monohydrate per dose. The amount of lactose in this medicine normally does not cause problems in people with lactose intolerance. The excipient lactose monohydrate contains small amounts of milk proteins, which may cause allergic reactions.
If your doctor has told you that you have an intolerance to some sugars, consult with them before taking this medicine.
3. How to use Aseriflu
Follow the instructions for using this medicine exactly as your doctor or pharmacist has told you. If you are unsure, ask your doctor or pharmacist again.
- Use this medicine every day until your doctor tells you to stop. Do not take more than the recommended dose. If you are unsure, ask your doctor or pharmacist.
- Do not stop taking this medicine or reduce the dose without talking to your doctor first.
- This medicine should be inhaled through the mouth into the lungs.
- You may not be able to taste or feel the powder on your tongue, even if you have used the inhaler correctly.
For asthma
Adults and adolescents 12 years and older
- Aseriflu 50 micrograms/250 micrograms - One inhalation twice a day
- Aseriflu 50 micrograms/500 micrograms - One inhalation twice a day
Use in children
Aseriflu is not recommended for use in children under 12 years of age.
For adults with Chronic Obstructive Pulmonary Disease (COPD)
- Aseriflu 50 micrograms/500 micrograms - One inhalation twice a day
Your symptoms may be well-controlled using Aseriflu twice a day. If this is the case, your doctor may decide to reduce your dose to once a day. The dose may change to:
- once at night if you have night-time symptoms,
- once in the morning if you have day-time symptoms.
It is very important that you follow your doctor's instructions about how many applications and how often you should take your medication.
If you are using Aseriflu to treat asthma, your doctor will want to regularly check your symptoms.
If your asthma gets worse or you have more difficulty breathing, see your doctor immediately.You may notice more wheezing or shortness of breath more often or that you need to use your rapid-acting rescue medication more frequently. If any of these things happen, you should continue using Aseriflu, but do not increase the number of applications. Your respiratory disease may worsen, and you could become seriously ill. See your doctor, as you may need additional treatment.
Instructions for use
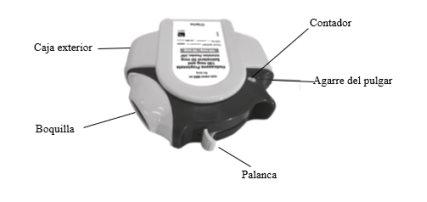
- Your doctor, nurse, or pharmacist should teach you how to use your inhaler. They should check how you use it from time to time. Not using Aseriflu correctly or as prescribed may result in your asthma or COPD not improving as it should.
- This inhaler contains blisters that contain Aseriflu powder.
- There is a dose counter on the top of the inhaler that shows how many doses are left. See Figure A. It counts down to 0. The numbers from 5 to 0 appear in red to warn you that there are few doses left. Once the counter shows 0, your inhaler is empty.

Figure A
Using your inhaler
- To open the Aseriflu inhaler, hold the outer case with one hand and place your thumb in the thumb grip with the other hand. Push your thumb away from you as far as it will go. Figure B. You will hear a "click". This will open a small hole in the mouthpiece.

Figure B
- Hold the device with the mouthpiece towards you. You can hold it with either your right or left hand. Slide the lever away from you. See Figure C. You will hear a "click". This will place the dose of medicine in the mouthpiece. Each time the lever is pulled back, a blister is opened, and the powder is prepared for inhalation. Do not play with the lever, as the blisters will open, and medicine will be wasted.

Figure C
- Hold the inhaler away from your mouth. Breathe out as much as you reasonably can. Do not breathe into the inhaler. See Figure D.

Figure D
- Place the mouthpiece in your lips; take a slow, deep breath in through the inhaler, not through your nose. See Figure E.
Take the inhaler out of your mouth.
Hold your breath for about 10 seconds or as long as you comfortably can. Breathe out slowly.
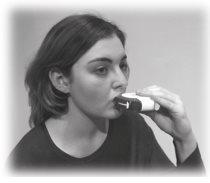
Figure E
- Afterward, rinse your mouth with water and spit it out and/or brush your teeth. This may help prevent mouth ulcers and hoarseness.
- To close the inhaler, slide the lever back towards you as far as it will go with your thumb. You will hear a "click".
The lever will automatically return to its original position. See Figure F. The inhaler is now ready to be used again.

Figure F
As with all inhalers, caregivers should ensure that children using Aseriflu use the inhalation technique correctly as described above.
Cleaning your inhaler
To clean it, wipe the mouthpiece of the Aseriflu inhaler with a dry cloth.
If you use more Aseriflu than you should
It is very important to use the inhaler exactly as you have been told. If you accidentally take a higher dose than recommended, talk to your doctor or pharmacist. You may notice that your heart beats faster than normal, and you may feel shaky. You may also feel dizzy, have a headache, feel weak, or have muscle pain or joint pain.
If you have used high doses for a long time, you should ask your doctor or pharmacist for advice. This is because high levels of Aseriflu may reduce the amount of steroid hormones produced by the adrenal gland.
In case of overdose, contact your doctor or pharmacist immediately or call the Toxicology Information Service, phone: 91 562 04 20, indicating the medicine and the amount administered.
If you forget to use Aseriflu
Do not take a double dose to make up for forgotten doses. Take the next dose at the usual time.
If you stop using Aseriflu
It is very important that you use Aseriflu every day as your doctor has told you. Keep taking it until your doctor tells you to stop. Do not stop using Aseriflu suddenly. This could make your breathing worse.
Also, if you stop taking Aseriflu suddenly or reduce your dose, you may (very rarely) experience problems with your adrenal gland (adrenal insufficiency), which can sometimes cause side effects.
These side effects may include any of the following:
- Stomach pain.
- Tiredness and loss of appetite, feeling unwell.
- Diarrhea.
- Weight loss.
- Headache or drowsiness.
- Low blood sugar levels.
- Low blood pressure and seizures (fits).
When your body is under stress, such as with fever, injury (e.g., car accident), infection, or surgery, adrenal insufficiency may worsen, and you may experience any of the side effects listed above.
If you experience any side effects, talk to your doctor or pharmacist. To prevent these symptoms, your doctor may prescribe an additional dose of corticosteroids in tablets during that time (such as prednisolone).
If you have any further questions about the use of this medicine, ask your doctor, pharmacist, or nurse.
4. Possible Adverse Effects
Like all medicines, this medicine can cause adverse effects, although not all people suffer from them. To reduce the appearance of adverse effects, your doctor will prescribe the lowest dose of Aseriflu that controls your asthma or Chronic Obstructive Pulmonary Disease (COPD).
Allergic Reactions: you may notice that your breathing suddenly worsens immediately after using Aseriflu.You may suffer from wheezing and coughing or shortness of breath. You may also notice itching, rash (urticaria), and swelling (usually of the face, lips, tongue, or throat). You may also suddenly feel that your heart is beating very fast, feel like you are losing consciousness, and feel dizzy (which can lead to collapse or loss of consciousness). If you experience any of these effects or if they appear suddenly after using Aseriflu, stop taking this medicine and inform your doctor immediately. Allergic reactions to Aseriflu are rare (affect less than 1 in 100 people).
Pneumonia (lung infection) in patients with COPD (frequent adverse effect)
Inform your doctorif you have any of the following symptoms while inhaling Aseriflu, they could be symptoms of a lung infection:
- Fever or chills
- Increased production of mucus, change in the color of mucus
- Increased coughing or increased difficulty breathing The following adverse effects are also listed:
Very Common (may affect more than 1 in 10 people)
- Headache, which usually improves with continued treatment.
- An increase in the number of colds has been reported in patients with Chronic Obstructive Pulmonary Disease (COPD).
Common (may affect up to 1 in 10 people)
- Candidiasis (itching, appearance of yellowish-white ulcers) in the mouth and throat. Also, pain in the tongue, hoarse voice, and throat irritation. Rinsing your mouth with water and spitting it out and/or brushing your teeth immediately after each dose of medicine may help. For the treatment of candidiasis, your doctor may prescribe antifungal medication (for the treatment of fungal infections).
- Pain, inflammation in the joints, and muscle pain.
- Muscle cramps.
The following adverse effects have been reported in patients with Chronic Obstructive Pulmonary Disease (COPD):
- Bruises and fractures.
- Inflammation of the sinuses (feeling of tension or congestion in the nose, cheeks, and behind the eyes, sometimes with a pulsating pain).
- Decreased potassium levels in the blood (you may feel irregular heartbeats, muscle weakness, cramps).
Uncommon (may affect up to 1 in 100 people)
- Increased blood sugar (glucose) levels (hyperglycemia). If you have diabetes, you will need to check your blood sugar levels more frequently and adjust your usual diabetic treatment if necessary.
- Cataracts (opacity of the eye lens).
- Very fast heart rate (tachycardia).
- Feeling tremors and a fast or irregular heartbeat (palpitations). These adverse effects are usually harmless and decrease when treatment is continued.
- Chest pain.
- Feeling of anxiety (occurs mainly in children).
- Sleep disorders.
- Allergic rash on the skin.
Rare (may affect up to 1 in 1,000 people)
- Difficulty breathing (or wheezing) that worsens immediately after using Aseriflu. If this happens, stop using this medicine. Use your rapid-acting "rescue" inhaler to improve your breathing and inform your doctor immediately.
- Aseriflu may increase the normal production of steroid hormones, particularly if you have been taking high doses for long periods. The effects include:
- Growth delay in children and adolescents.
- Decreased bone mineral density.
- Glaucoma.
- Weight gain.
- Round face (moon face) (Cushing's syndrome).
Your doctor will regularly monitor any of these adverse effects and ensure that you are taking the lowest dose of Aseriflu to control your asthma.
- Changes in behavior, such as hyperactivity and irritability (these effects occur mainly in children).
- Irregular heartbeats or extra heartbeats (arrhythmias). Consult your doctor, but do not stop taking Aseriflu unless your doctor tells you to do so.
- Fungal infection in the esophagus (throat), which can cause difficulty swallowing.
Frequency Not Known (frequency cannot be estimated from available data)
- Depression or aggression. These effects are more likely to occur in children.
- Blurred vision
Reporting Adverse Effects
If you experience any type of adverse effect, consult your doctor or pharmacist, even if it is a possible adverse effect that is not listed in this leaflet. You can also report them directly through the Spanish Pharmacovigilance System for Human Use Medicines: www.notificaRAM.es.
By reporting adverse effects, you can contribute to providing more information on the safety of this medicine.
5. Storage of Aseriflu
- Keep this medicine out of the sight and reach of children.
- Do not use this medicine after the expiration date that appears on the label and on the carton after CAD. The expiration date is the last day of the month indicated.
- Do not refrigerate or freeze.
- Once the aluminum bag has been opened, the medicine it contains must be used within the following 2 months. Once opened, store the inhaler below 25 °C
- Medicines should not be thrown away through wastewater or household waste. Deposit the containers and medicines you no longer need at the SIGRE collection point in the pharmacy. Ask your pharmacist how to dispose of the containers and medicines you no longer need. This way, you will help protect the environment.
6. Package Contents and Additional Information
Aseriflu Composition
- The active ingredients are salmeterol and fluticasone propionate. Each individual inhalation provides a released dose (the dose that comes out of the mouthpiece) of 47 micrograms of salmeterol (as salmeterol xinafoate) and 231 or 460 micrograms of fluticasone propionate. This corresponds to a pre-dispensed dose of 50 micrograms of salmeterol (as salmeterol xinafoate) and 250 or 500 micrograms of fluticasone propionate.
- The other component is lactose monohydrate (contains milk proteins). See section 2.
Product Appearance and Package Contents
- Aseriflu is supplied as a disposable red and white plastic inhaler containing a strip of blisters with 60 blisters arranged at regular intervals. The blister contains a white to off-white powder. The inhaler is contained in a laminated aluminum bag.
- Each dose is pre-dispensed.
This medicine is available in packages containing 1 inhaler. Each inhaler contains 60 inhalations.
Marketing Authorization Holder and Manufacturer
Marketing Authorization Holder
Cipla Europe NV
De Keyserlei 58-60, Box-19,
2018, Antwerp,
Belgium
Manufacturer
Cipla Europe NV
De Keyserlei 58-60, Box-19,
2018, Antwerp,
Belgium
This medicine is authorized in the Member States of the European Economic Area under the following names:
Sweden: Salmeterol/Flutikason ELC Group 50 mikrogram/250 mikrogram/ inhalation powder, pre-dispensed dose
Salmeterol/Flutikason ELC Group 50 mikrogram/500 mikrogram/ inhalation powder, pre-dispensed dose
Norway: Salmeterol/flutikason ELC 50 mikrogram/250 mikrogram per dose inhalation powder, pre-dispensed
Salmeterol/flutikason ELC 50 mikrogram/500 mikrogram per dose inhalation powder, pre-dispensed
Italy: Salmeterolo e Fluticasone Teva Italia 50 microgrammi/250 microgrammi per dose, powder for inhalation, pre-dispensed
Salmeterolo e Fluticasone Teva Italia 50 microgrammi/500 microgrammi per dose, powder for inhalation, pre-dispensed
France: PROPIONATE DE FLUTICASONE/SALMETEROL ELC CIPHALER 250 microgrammes/50 microgrammes/dose, powder for inhalation in a single-dose container
PROPIONATE DE FLUTICASONE/SALMETEROL ELC CIPHALER 500 microgrammes/50 microgrammes/dose, powder for inhalation in a single-dose container
Poland: Fullhale Ciphaler 50 mikrogramów/250 mikrogramów/dawke proszek do inhalacji, podzielony
Fullhale Ciphaler 50 mikrogramów/500 mikrogramów/dawke proszek do inhalacji, podzielony
Austria: Salmeterol/Fluticason G.L. 50 Mikrogramm/250 Mikrogramm-einzeldosiertes Pulver zur Inhalation
Salmeterol/Fluticason G.L. 50 Mikrogramm/500 Mikrogramm-einzeldosiertes Pulver zur Inhalation
Romania: Zoreeda Ciphaler 50 micrograme/250 micrograme pulbere de inhalat
Zoreeda Ciphaler 50 micrograme/500 micrograme pulbere de inhalat
Belgium: Fullhale Ciphaler 50 microgram/250 microgram/dosis inhalatiepoeder, voorverdeeld
Fullhale Ciphaler 50 microgram/500 microgram/dosis inhalatiepoeder, voorverdeeld
Czech Republic: Zoreeda Ciphaler 50 mikrogramu/250 mikrogramu dávkovaný prášek k inhalaci
Zoreeda Ciphaler 50 mikrogramu/500 mikrogramu dávkovaný prášek k inhalaci
Slovakia: Zoreeda Ciphaler 50 mikrogramov/250 mikrogramov dávkovaný inhalacný prášok
Zoreeda Ciphaler 50 mikrogramov/500 mikrogramov dávkovaný inhalacný prášok
Denmark: Zoreeda Ciphaler 50 mikrogram/250 mikrogram/dosis inhalationspulver i diskos
Zoreeda Ciphaler 50 mikrogram/500 mikrogram/dosis inhalationspulver i diskos
Spain: Aseriflu 50 micrograms/250 micrograms/inhalation powder for inhalation (single dose)
Aseriflu 50 micrograms/500 micrograms/inhalation powder for inhalation (single dose)
Date of the Last Revision of this Leaflet:September 2024
Detailed information about this medicine is available on the website of the Spanish Agency for Medicines and Health Products (AEMPS) http://www.aemps.gob.es/
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to ASERIFLU 50/500 micrograms inhalation powder (single dose)Dosage form: PULMONARY INHALATION, 50 micrograms/250 microgramsActive substance: salmeterol and fluticasoneManufacturer: Sandoz Farmaceutica S.A.Prescription requiredDosage form: PULMONARY INHALATION, 50 micrograms/500 microgramsActive substance: salmeterol and fluticasoneManufacturer: Sandoz Farmaceutica S.A.Prescription requiredDosage form: PULMONARY INHALATION, 50 micrograms / 100 microgramsActive substance: salmeterol and fluticasoneManufacturer: Zentiva K.S.Prescription required
Online doctors for ASERIFLU 50/500 micrograms inhalation powder (single dose)
Discuss questions about ASERIFLU 50/500 micrograms inhalation powder (single dose), including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions















