
BRAQUIX 25/250 micrograms/inhalation, pressurized inhalation suspension

How to use BRAQUIX 25/250 micrograms/inhalation, pressurized inhalation suspension
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the Patient
Braquix 25 micrograms/250 micrograms/inhalation, suspension for inhalation in a pressurized container
Salmeterol/Fluticasone Propionate
Read the entire package leaflet carefully before starting to use this medication, as it contains important information for you.
- Keep this package leaflet, as you may need to read it again. If you have any questions, consult your doctor or pharmacist.
- This medication has been prescribed to you only, and you should not give it to others, even if they have the same symptoms as you, as it may harm them.
- If you experience side effects, consult your doctor or pharmacist, even if they are not listed in this package leaflet. See section 4.
Contents of the Package Leaflet
- What is Braquix and what is it used for
- What you need to know before starting to use Braquix
- How to use Braquix
- Possible side effects
- Storage of Braquix
- Contents of the container and additional information
1. What is Braquix and what is it used for
Braquix contains two active substances: salmeterol and fluticasone propionate:
- Salmeterol is a long-acting bronchodilator. Bronchodilators help keep the airways in the lungs open, making it easier to breathe in and out. The effects last for at least 12 hours.
- Fluticasone propionate is a corticosteroid that reduces inflammation and irritation in the lungs.
Your doctor has prescribed this medication to help prevent respiratory problems such as asthma.
You should use Braquix every day, as your doctor has recommended. This will ensure that the medication works correctly to control your asthma.
Salmeterol/Fluticasone helps prevent shortness of breath and wheezing. However, Salmeterol/Fluticasone should not be used to relieve a sudden attack of shortness of breath or wheezing. If this happens, you should use your "rescue" medication with rapid action, such as salbutamol. You should always carry your rapid-action rescue medication with you.
2. What you need to know before starting to use Braquix
Do not take Braquix:
- If you are allergic to salmeterol, fluticasone propionate, or any of the other components of this medication (listed in section 6).
Warnings and Precautions
Consult your doctor or pharmacist before taking Salmeterol/Fluticasone if you have:
- Heart problems, including rapid or irregular heartbeat;
- Hyperthyroidism;
- High blood pressure;
- Diabetes mellitus (Salmeterol/Fluticasone may increase your blood sugar levels);
- Low potassium levels in the blood;
- Tuberculosis (TB) currently or in the past, or other lung infections;
Contact your doctor if you experience blurred vision or other visual disturbances.
Other Medications and Braquix
Tell your doctor or pharmacist if you are taking, have recently taken, or may take any other medication, including medications for asthma or any medication obtained without a prescription. This is because Salmeterol/Fluticasone may not be suitable to be taken with some other medications.
Tell your doctor if you are taking the following medications before starting to use Salmeterol/Fluticasone:
- Beta-blockers (such as atenolol, propranolol, and sotalol). Beta-blockers are mainly used to treat high blood pressure or other heart conditions.
- Medications to treat infections (such as ketoconazole, itraconazole, and erythromycin), including some medications for the treatment of HIV (such as ritonavir, products containing cobicistat). Some of these medications may increase the amount of fluticasone propionate or salmeterol in your body. This may increase your risk of experiencing side effects with this medication, including irregular heartbeats, or may worsen side effects. Therefore, your doctor may monitor you closely if you are taking these medications.
- Corticosteroids (oral or injectable). If you have taken these medications recently, this may increase the risk that this medication will affect your adrenal gland.
- Diuretics, also known as water pills, used to treat high blood pressure.
- Other bronchodilators (such as salbutamol).
- Medications with xanthine. They are often used to treat asthma.
Pregnancy and Breastfeeding
If you are pregnant or breastfeeding, think you may be pregnant, or plan to become pregnant, consult your doctor or pharmacist before using this medication.
Driving and Using Machines
Salmeterol/Fluticasone is unlikely to affect your ability to drive or use machines.
3. How to Use Salmeterol/Fluticasone Genetic
Follow the instructions for administration of this medication exactly as indicated by your doctor or pharmacist. If you are unsure, consult your doctor or pharmacist again.
- Use Salmeterol/Fluticasone every day, until your doctor tells you to stop. Do not take more than the recommended dose. If you are unsure, consult your doctor or pharmacist.
- Do not stop taking this medication or reduce the dose of Salmeterol/Fluticasone without talking to your doctor first.
- Salmeterol/Fluticasone should be inhaled through the mouth into the lungs.
- Use Salmeterol/Fluticasone, always keeping track of the applications.
- The inhaler has been designed to perform 120 applications.
However, it is not possible to know when the inhaler is empty and when the 120 applications have been released. It is possible that a small amount of liquid may still be left in the container.
Please make sure to replace your inhaler after 120 applications so that you can be sure you are receiving the correct amount of medication in each application.
Adults and Adolescents from 12 Years Old
- Salmeterol/Fluticasone Genetic 25/250 - 2 applications twice a day
Children from 4 to 12 Years Old
- Salmeterol/Fluticasone Genetic 25/50 - 2 applications twice a day
- Salmeterol/Fluticasone Genetic is not recommended for use in children under 4 years old.
Your symptoms may be well-controlled using Salmeterol/Fluticasone twice a day. If this is the case, your doctor may decide to reduce your dose to once a day. The dose may change to:
- once at night if you have nighttimesymptoms.
- once in the morning if you have daytimesymptoms.
It is very important that you follow your doctor's instructions on how many applications and how often you should administer them.
If you are using this medication to treat asthma, your doctor will want to monitor your symptoms regularly.
If your asthma worsens or you have more difficulty breathing, see your doctor immediately.You may notice more wheezing or shortness of breath, chest tightness, or that you need to use your rapid-action rescue medication more frequently. If any of these things happen to you, you should continue using Salmeterol/Fluticasone Genetic, but do not increase the number of applications. Your respiratory disease may worsen and become seriously ill. See your doctor, as you may need additional treatment.
Instructions for Use
- Your doctor, nurse, or pharmacist should teach you how to use the inhaler. Periodically, they should check how you use it. Not using Salmeterol/Fluticasone Genetic inhaler properly, or as prescribed, may result in your asthma not improving as it should.
- The medication is in a pressurized cartridge inside a plastic casing with a mouthpiece.
- A new, complete inhaler has enough medication for a minimum of 120 applications. After 120 applications, it is possible that the inhaler may no longer have enough medication to administer a full dose of medication.
Checking the Inhaler
- If you are using your inhaler for the first time, check that it works properly. Remove the mouthpiece cover by squeezing it gently on the sides with your thumb and index finger and pulling it off.
- To ensure the inhaler works, shake it well, direct the mouthpiece away from you, and press the cartridge to release an application into the air. Repeat these steps, shaking the inhaler before releasing a second application into the air. The total number of applications released into the air, before using the inhaler, should be two.
- After these two initial test applications, you can start using your inhaler.
- If you have not used your inhaler for a week or more, or if your inhaler is very cold (below 0°C), release two applications of medication into the air.
Warnings
Never separate the metal cartridge from the inhaler.
Only if the inhaler becomes very cold (below 0°C), remove the metal cartridge from the plastic casing and warm it in your hands for a few minutes before using it. Never use anything else to warm it. Once warmed and before using it, press the cartridge firmly to release two applications into the air.
Using the Inhaler
It is essential to start inhaling as slowly as possible just before using your inhaler.
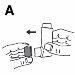
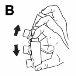
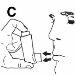
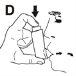
- It is recommended that you stand or sit upright while using the inhaler.
- Remove the mouthpiece cover (Figure A).
- Check that the mouthpiece is clean and free of dust or any foreign particles, both inside and outside.
- Shake the inhaler 4 or 5 times to ensure the contents of the inhaler are mixed evenly (Figure B).
- Hold the inhaler in a vertical position with your thumb on the base of the inhaler, below the mouthpiece. Expel as much air as possible (Figure C).
- Place the mouthpiece in your mouth, between your teeth. Close your lips around the mouthpiece. Do not bite it.
- Take a slow and deep breath in through your mouth. Immediately after starting to breathe in, press the top of the inhaler firmly to release an application of medication. Do this while continuing to breathe in slowly and steadily (Figure D).
- Hold your breath, remove the inhaler from your mouth, and your finger from the top of the inhaler. Continue holding your breath for a few seconds, as long as you can.
- Wait approximately half a minute between each application of medication and then repeat steps 4 to 8.
- Afterward, rinse your mouth with water and spit it out and/or brush your teeth. This will help prevent the appearance of mouth ulcers and hoarseness.
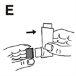
- After using it, always put the mouthpiece cover back on immediately, to protect it from dust (Figure E). When the mouthpiece cover is put back on correctly, it will make a "click". Do not use excessive force.
Take your time in steps 5, 6, 7, and 8. It is essential that you start inhaling as slowly as possible just before using your inhaler.
The first few times you use the inhaler, you should use it in front of a mirror. If you see "mist" coming out of the top of the inhaler or the sides of your mouth, you should start again from step 4.
As with all inhalers, caregivers should ensure that children using Salmeterol/Fluticasone use the correct inhalation technique described above.
If you or your child find it difficult to use the inhaler, your doctor, nurse, or pharmacist may recommend that you use an AeroChamber Plus® spacer device with your inhaler. Your doctor, nurse, pharmacist, or other healthcare professional should show you how to use the spacer device with your inhaler and how to maintain your spacer device, and answer any questions you may have. If you are using the spacer device with your inhaler, it is essential that you do not stop using it without consulting your doctor or nurse first. It is not recommended to use other spacer devices with Salmeterol/Fluticasone Genetic, and you should not change from the AeroChamber Plus® to another device.It is also essential that you do not change or stop using the spacer without consulting your doctor. He will know how to modify the therapy. Always consult your doctor before making any changes to your asthma treatment.
Some children or people with little hand strength may find it easier to hold the inhaler with both hands. Place your two index fingers on the top of the inhaler and your thumbs on the base, below the mouthpiece.
Cleaning the Inhaler
To prevent your inhaler from becoming blocked, it is essential to clean it at least once a week.
To clean your inhaler:
- Remove the mouthpiece cover.
- Never remove the metal cartridge from the plastic casing.
- Clean the inside and outside of the mouthpiece and the plastic casing with a dry cloth or a paper towel.
- Put the mouthpiece cover back on. It will make a "click" when it is put back on correctly.
- Do not use excessive force.
- Do not put the metal cartridge in water.
If You Use More Salmeterol/Fluticasone Genetic Than You Should
It is essential to use the inhaler exactly as indicated. If you accidentally take a higher dose than recommended, consult your doctor or pharmacist. You may notice that your heart beats faster than usual and feel tremors. You may also have a headache, dizziness, muscle weakness, and joint pain.
If you have used high doses for extended periods, you should ask your doctor or pharmacist for advice. This is because high concentrations of Salmeterol/Fluticasone may reduce the amount of steroid hormones produced by the adrenal gland.
In case of overdose or accidental ingestion, consult your doctor or call the Toxicology Information Service, phone 91 562 04 20, indicating the medication and the amount ingested.
If You Forget to Use Salmeterol/Fluticasone Genetic
Do not take a double dose to make up for forgotten doses. Take the next dose at the usual time.
If You Interrupt Treatment with Salmeterol/Fluticasone Genetic
It is very important that you take your Salmeterol/Fluticasone every day as indicated. Continue taking it until your doctor tells you to stop. Do not interrupt or suddenly stop your treatment with Salmeterol/Fluticasone. This could make your breathing worse.
Additionally, if you suddenly stop taking this medication or reduce your dose of Salmeterol/Fluticasone, you may (very rarely) experience problems with your adrenal gland (adrenal insufficiency), which can sometimes cause side effects.
These side effects may include any of the following:
- Abdominal pain
- Fatigue and loss of appetite, feeling unwell
- Nausea and diarrhea
- Weight loss
- Headache or drowsiness
- Low blood sugar levels
- Low blood pressure and seizures (fits)
When your body is under stress, such as fever, trauma (such as a car accident), infection, or surgery, adrenal insufficiency may worsen, and you may experience any of the above side effects.
If you experience any side effects, consult your doctor or pharmacist. To prevent these symptoms, your doctor may prescribe an additional dose of corticosteroids in tablets (such as prednisolone).
If you have any further questions about the use of this medication, ask your doctor, pharmacist, or nurse.
4. Possible Side Effects
Like all medications, this medication can cause side effects, although not everyone experiences them. To reduce the possibility of side effects, your doctor will prescribe the lowest dose of Salmeterol/Fluticasone necessary to control your asthma.
Allergic Reactions: You may notice that your breathing suddenly worsens immediately after using Salmeterol/Fluticasone
The following are other side effects:
Very Common (may affect more than 1 in 10 people)
- Headache: usually improves with treatment.
- An increase in the number of colds has been reported in patients with COPD.
Frequent (may affect up to 1 in 10 people)
- Thrush (painful, yellowish-white patches, with relief) in the mouth and throat. Also, tongue pain and hoarse voice and throat irritation. Rinsing the mouth with water and spitting it out immediately and/or brushing your teeth immediately after each dose of medication may help. For the treatment of thrush, your doctor may prescribe antifungal medication.
- Pain and inflammation of the joints and muscle pain.
- Muscle cramps.
The following adverse effects have been reported in patients with Chronic Obstructive Pulmonary Disease (COPD):
- Pneumonia and bronchitis (lung infection). Inform your doctor if you notice any of the following symptoms: increased production of sputum, change in the color of sputum, fever, chills, increased cough, increased difficulty breathing.
- Bruises and fractures.
- Inflammation of the paranasal sinuses (feeling of tension or fullness in the nose, cheeks, and behind the eyes, sometimes with a pulsating pain).
- Decreased levels of potassium in the blood (may feel irregular heartbeats, muscle weakness, cramps).
Infrequent (may affect up to 1 in 100 people)
- Increased amount of sugar (glucose) in the blood (hyperglycemia). If you have diabetes, it will be necessary to monitor your blood sugar levels more frequently and adjust your usual diabetic treatment if necessary.
- Cataracts (opacity of the eye lens).
- Very fast heart rate (tachycardia).
- Feeling tremors and a fast or irregular heart rate (palpitations). These adverse effects are usually harmless and decrease when treatment is continued.
- Chest pain.
- Feeling of concern (occurs mainly in children).
- Sleep disorders.
- Allergic skin rash.
Rare (may affect up to 1 in 1,000 people)
- Breathing difficulties or wheezing that worsen immediately after using salmeterol/fluticasone.If this happens, stop using this medication. Use your rapid-acting "rescue" inhaler to improve your breathing and inform your doctor immediately.
- Salmeterol/fluticasone may increase the normal production of steroid hormones, particularly if you have been taking high doses for long periods of time.
The effects include:
- Slowing of growth in children and adolescents
- Decreased bone mineral density
- Glaucoma
- Weight gain
- Round face (moon face) (Cushing's syndrome)
Your doctor will regularly monitor any of these adverse effects and ensure that you are taking the lowest dose of salmeterol/fluticasone to control your asthma.
- Changes in behavior, such as hyperactivity and irritability (these effects occur mainly in children).
- Irregular heartbeats or the heart skips a beat (arrhythmias). Consult your doctor, but do not stop taking salmeterol/fluticasone unless your doctor tells you to do so.
- Fungal infection in the esophagus (throat), which can cause difficulty swallowing.
Frequency not known (frequency cannot be estimated from available data):
- Depression or aggression. These effects are more likely in children.
- Blurred vision
Reporting of adverse effects
If you experience any type of adverse effect, consult your doctor or pharmacist, even if it is a possible adverse effect that does not appear in this prospectus. You can also report them directly through the Spanish Pharmacovigilance System for Human Use Medicines: www.notificaRAM.es. By reporting adverse effects, you can contribute to providing more information on the safety of this medication.
5. Conservation of Braquix
Keep this medication out of sight and reach of children.
Do not use Braquix after the expiration date that appears on the label and on the carton after CAD. The expiration date is the last day of the month indicated.
Do not store at a temperature above 25°C.
The cartridge contains a pressurized liquid. Do not expose it to temperatures above 50°C, protect it from direct sunlight. Do not puncture, break, or burn the cartridge even if it is empty.
Do not use this medication if you observe visible signs of deterioration.
As with most medications administered by inhalation in pressurized cartridges, the therapeutic effect may decrease if the cartridge is cooled.
Medicines should not be thrown down the drain or into the trash. Deposit the containers and medications you no longer need in the Sigre Point of the pharmacy. In case of doubt, ask your pharmacist how to dispose of the containers and medications you no longer need. This way, you will help protect the environment.
6. Appearance of the product and package contents
Composition of Braquix
The active ingredients are salmeterol and fluticasone propionate. Each pressurized dose contains 25 micrograms of salmeterol (as salmeterol xinafoate) and 250 micrograms of fluticasone propionate.
The other ingredient is the propellant: norflurane (HFA 134a).
Appearance of the product and package contents
- Braquix is presented in a pressurized container with a counter, which releases the medication in the form of a suspension for inhalation through the mouth to the lungs.
- The pressurized cartridge contains a homogeneous white inhalation suspension.
- The cartridge is located inside a plastic device that incorporates a mouthpiece and a violet-colored dust protector.
- Each carton contains 1 inhaler. Each inhaler contains 120 applications.
Marketing authorization holder
MABO-FARMA, S.A.
Calle Vía de los Poblados 3, Edificio 6,
28033 Madrid,
Spain.
Manufacturer
GENETIC, S.P.A.
Contrada Canfora
Fisciano, Salerno
84084 Italy
This medication is authorized in the member states of the European Economic Area with the following names:
Country | Medication name |
Portugal: | Flusonide |
Spain: | Braquix 25 micrograms/250 micrograms/inhalation, inhalation suspension in pressurized container. |
Date of the last revision of this prospectus:March 2022
Detailed and updated information on this medication is available on the website of the Spanish Agency for Medicines and Health Products (AEMPS) http://www.aemps.gob.es/
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to BRAQUIX 25/250 micrograms/inhalation, pressurized inhalation suspensionDosage form: PULMONARY INHALATION, 50 micrograms/250 microgramsActive substance: salmeterol and fluticasoneManufacturer: Sandoz Farmaceutica S.A.Prescription requiredDosage form: PULMONARY INHALATION, 50 micrograms/500 microgramsActive substance: salmeterol and fluticasoneManufacturer: Sandoz Farmaceutica S.A.Prescription requiredDosage form: PULMONARY INHALATION, 50 micrograms / 100 microgramsActive substance: salmeterol and fluticasoneManufacturer: Zentiva K.S.Prescription required
Online doctors for BRAQUIX 25/250 micrograms/inhalation, pressurized inhalation suspension
Discuss questions about BRAQUIX 25/250 micrograms/inhalation, pressurized inhalation suspension, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions















