
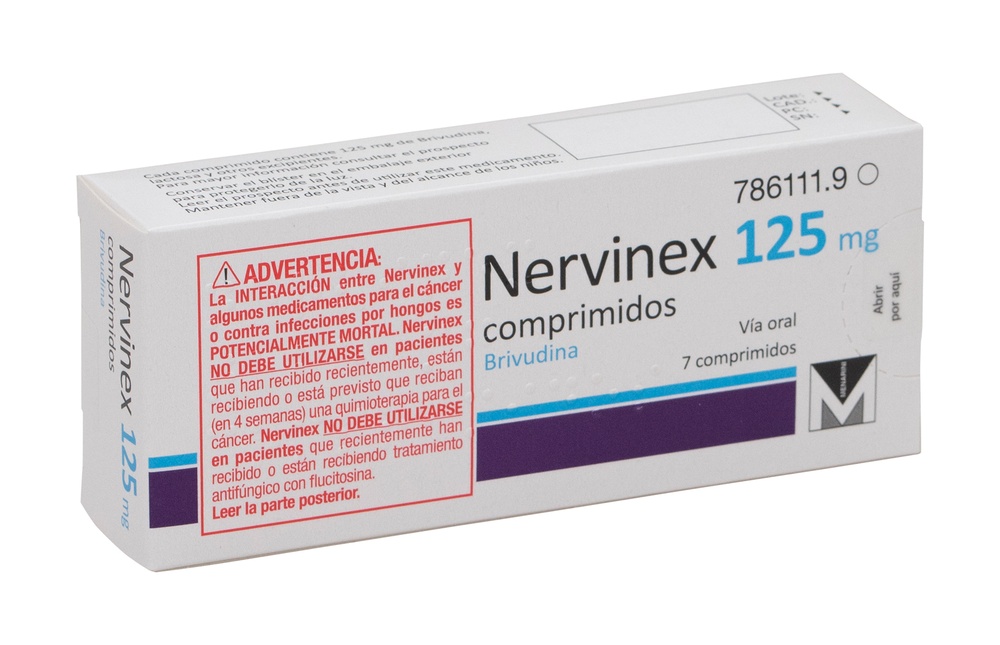
NERVINEX 125 mg TABLETS

How to use NERVINEX 125 mg TABLETS
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Leaflet: information for the patient
Nervinex 125 mg tablets
Brivudine
DO NOT TAKE Nervinex (BRIVUDINE)IF you have recently received, are receiving or are scheduled to receive (within 4 weeks) cancer chemotherapy or antineoplastic therapy. DO NOT TAKE Nervinex IF YOU HAVE A FUNGAL INFECTION and have recently received or are receiving antifungal treatment with flucytosine (see section 2, including the red box). The INTERACTIONbetween Nervinex (brivudine) and some cancer treatments or flucytosine is POTENTIALLY FATAL.
Read the entire leaflet carefully before starting to take this medicine, as it contains important information for you.
- Keep this leaflet, as you may need to read it again.
- If you have any questions, ask your doctor or pharmacist or nurse.
- This medicine has been prescribed to you only, and you should not give it to others, even if they have the same symptoms as you, as it may harm them.
- If you experience side effects, ask your doctor or pharmacist or nurse, even if they are side effects not listed in this leaflet. See section 4.
Contents of the leaflet:
- What is Nervinex and what is it used for
- What you need to know before taking Nervinex
- How to take Nervinex
- Possible side effects
- Storage of Nervinex
- Package contents and additional information
1. What is Nervinex and what is it used for
Nervinex contains the active substance brivudine. Nervinex has an antiviral effect and stops the multiplication of the virus that causes herpes (varicella-zoster virus).
Nervinex is used for the early treatment of herpes infection (herpes zoster) in adults without immune system disorders (the body's defenses).
2. What you need to know before taking Nervinex
DO NOT TAKE Nervinex:
- if you have recently received, are receiving or are scheduled to receive (within 4 weeks) antineoplastic chemotherapy (e.g., capecitabine, 5-fluorouracil (5-FU), tegafur, etc.) (see red box and section "Other medicines and Nervinex")
- if you have a fungal infection and have recently received or are receiving antifungal treatment with flucytosine (see red box and section "Other medicines and Nervinex")
- if you are allergic (hypersensitive) to the active substance brivudine
- if you are allergic (hypersensitive) to any of the other components of Nervinex (see section 6)
- if you are pregnant or breastfeeding
- if you are under 18 years old
DO NOT TAKE Nervinex: ? if you have recently received,are receivingor are scheduled to receive (within 4 weeks) antineoplastic chemotherapy(especially capecitabine, 5-fluorouracil (5-FU) or other fluoropyrimidines by mouth or by injection or locally in the form of creams, ointments, eye drops or any other type of externally applied medicine) ? if you have a fungal infection and have recently received or are receiving antifungal treatment with flucytosine ? if you have recently used, are usingor are scheduled to use (within 4 weeks) a medicine for warts or against actinic keratosis or Bowen's disease that contains fluoropyrimidines (5-fluorouracil or others) ? if your immune system (i.e., your body's defenses against infections) is severely impaired; for example, if you have recently received or are receiving:
|
Warnings and precautions
Do not take Nervinex andconsult your doctor or pharmacist:
- if you have recently received, are receiving or are scheduled to receive (within 4 weeks) antineoplastic chemotherapy (by mouth or by injection or locally in the form of creams, ointments, eye drops or any other type of externally applied medicine)
- if you have a fungal infection and have recently received or are receiving antifungal treatment with flucytosine (see sections "DO NOT TAKE Nervinex" in the red box, and "Use of Nervinex with other medicines").
Do not take Nervinex if your skin rashis very advanced (start of crusting). If in doubt, consult your doctor.
Consult your doctor before taking Nervinex if you have any chronic liver disease(such as chronic hepatitis).
You should not take Nervinex for more than 7 days, as extending treatment beyond the recommended 7 days increases the risk of developing hepatitis (see also section 4).
Children and adolescents
Do not give Nervinex to children and adolescents between 0 and 18 years old, as safety and efficacy have not been studied in this age group.
Use of Nervinex with other medicines
Before starting treatment with Nervinex, tell your doctor or pharmacist if you are using, have recently used or may need to use any other medicine, including those purchased without a prescription. This is extremely important, as Nervinex may enhance the toxic effect of other medicines.
WARNING:
Special warning for patients withantineoplastic chemotherapy or fungal infections(see also the previous red box):
Nervinex should not be used in patientswho have recently received, are receiving or are scheduled to receive (within 4 weeks) certain cancer chemotherapy or antineoplastic therapy. The harmful effects of these medicines (fluoropyrimidines) could increase greatly and be fatal.
- 5-fluorouracil (5-FU), including local use forms
- capecitabine
- tegafur
- other 5-fluoropyrimidines
- combinations of some of the active substances mentioned with other active ingredients
Nervinex should not be used at the same time as medicines containing the active ingredient flucytosine used to treat fungal infections.
Do not take Nervinex and consult your doctor immediately:
- if you have recently received, are receiving or are scheduled to receive (within 4 weeks) any of the aforementioned medicines
- if you have recently received or are receiving antifungal treatment with flucytosine
If you have taken Nervinex and one of the aforementioned medicines by mistake:
- stop taking both medicines
- consult a doctor immediately
- go to the hospital for immediate treatment(to protect you from systemic infections and dehydration).
The symptoms and signs of 5-fluorouracil toxicity (and other fluoropyrimidines), due to the aforementioned interactions, include:
- dizziness; diarrhea; inflammation of the mouth and/or mouth mucosa; fatigue, increased sensitivity to infections, tiredness (decrease in white blood cell count and decrease in bone marrow function); red rash all over the body, with sensitive skin to the touch, which evolves into large blisters that progress to extensive areas of peeled skin (toxic epidermal necrolysis) (see also section 4).
Post-marketing experience indicates a possible interaction between brivudine and dopaminergic medicines for the treatment of Parkinson's disease, which may facilitate the appearance of chorea (abnormal and involuntary movements, similar to those of a dance, especially of arms, legs, and face).
Taking Nervinex with food and drinks
You can take Nervinex with or without food.
Pregnancy and breastfeeding
Consult your doctor or pharmacist before using any medicine.
You should not take Nervinex during pregnancy.
You should not take Nervinex if you are breastfeeding. The active ingredient of Nervinex may pass to the baby through breast milk.
Driving and using machines
Although rare, some patients who have taken Nervinex have suffered from dizziness and drowsiness. If you notice these side effects, refrain from driving, using machines, or working without being in a safe position. Ask your doctor for advice.
Nervinex contains lactose
This medicine contains lactose. If your doctor has told you that you have an intolerance to certain sugars, consult with them before taking this medicine.
3. How to take Nervinex
Follow the administration instructions of this medicine exactly as indicated by your doctor. If in doubt, consult your doctor or pharmacist again.
The recommended dose is:
1 tablet of 125 mg of Nervinex once a day for 7 days.
Take the Nervinex tablet every day at approximately the same time.
You can take Nervinex with or without food.
Swallow the tablet whole with enough liquid, for example, a glass of water.
You should start treatment as soon as possible.This means that, if possible, you should start taking Nervinex:
- within 3 days of the first symptoms of herpes on the skin (skin rash) or
- within 2 days of the first blisters.
Complete the 7-day treatment cycle even if you feel better beforehand.
If your symptoms persist or worsen during the week of treatment, you should consult your doctor.
Taking the normal dose of Nervinex reduces the risk of developing postherpetic neuralgia in patients over 50 years old. Postherpetic neuralgia is a persistent pain that appears in the area affected by herpes, after the improvement of the skin rash.
Treatment duration
This medicine is for short-term use. It should be administered only for 7 days. Do not take a second treatment cycle.
Use in children and adolescents
Do not take Nervinex if you are under 18 years old.
If you take more Nervinex tablets than you should
Inform a doctor if you take more tablets than you should. They will decide if it is necessary to take additional measures.
If you forget to take Nervinex
If you forget to take a tablet at the usual time, take it as soon as you remember. The next day, take the corresponding tablet at approximately the same time as the previous day. Maintain this new dose rhythm until you complete the 7-day treatment cycle.
Do not take a double dose to make up for forgotten doses.
Inform your doctor if you have forgotten to take the daily dose of the medicine repeatedly.
If you stop taking Nervinex
Do not stop taking Nervinex without consulting your doctor first. To get the maximum benefit from this treatment, you should take it for 7 days.
If you have any other questions about the use of this medicine, ask your doctor or pharmacist.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Stop taking Nervinex and inform your doctor immediately if you have an allergic reaction with signs and symptomsthat include itching or redness of the skin (rash), increased sweating, swelling (of hands, feet, tongue, lips, eyelids, or larynx), difficulty breathing (see also section 4). These symptoms could be serious and require urgent medical attention.
The following side effects are common (may affect up to 1 in 10 people):
- nausea (feeling sick)
The following side effects are uncommon (may affect up to 1 in 100 people):
- a decrease in the number of a type of white blood cells (granulocytes)
- a decrease in the number of red blood cells (anemia)
- allergic reactions that include:
- skin itching (pruritus)
- redness of the skin (erythematous rash)
- increased sweating
- swelling of hands, feet, face, tongue, lips, eyelids, or larynx (laryngeal edema)
- cough, difficulty breathing and/or shortness of breath
- loss of appetite
- anxiety
- insomnia, drowsiness
- headache
- dizziness
- vertigo (feeling of spinning)
- abnormal sensations, e.g., burning, pinching, tingling, feeling of pins and needles under the skin, mostly in arms and legs (paresthesia)
- high blood pressure
- indigestion (dyspepsia), vomiting, stomach pain
- diarrhea
- excess gas in the stomach or intestine (flatulence)
- constipation
- chronic liver disease with fat accumulation (fatty liver)
- an increase in blood levels of certain substances produced by the liver (increase in liver enzymes)
- weakness, tiredness (fatigue)
- flu-like symptoms (malaise, fever, generalized pain, and chills)
The following side effects are rare (may affect up to 1 in 1,000 people):
- low blood pressure
- decrease in the number of platelets in the blood
- hallucinations, delirium
- confusion
- tremor
- alteration of taste
- ear pain
- inflammation of the liver (hepatitis), increase in bilirubin in the blood
- bone pain
The following side effects have also been reported, although their frequency is not known (the frequency cannot be estimated from the available data):
- balance disorder
- inflammation of blood vessels (vasculitis)
- sudden onset liver failure
- localized skin inflammation that reappears in the same place after a while (fixed eruption), skin inflammation with peeling (exfoliative dermatitis), severe generalized rash on the skin and inside the mouth, due to an allergic reaction (erythema multiforme), skin ulcers, mouth, eyes, and genital areas (Stevens-Johnson syndrome).
- restlessness
- mood change
- depressive mood
- feeling of aggression, agitation, anxiety
- fainting
Reporting of side effects
If you experience any side effects, consult your doctor or pharmacist, even if they are possible side effects not listed in this leaflet. You can also report them through the Spanish Pharmacovigilance System for Human Use Medicines: www.notificaRAM.es. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of Nervinex
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date stated on the carton and blister. The expiry date is the last day of the month indicated.
Keep the blister in the outer packaging to protect it from light.
Medicines should not be disposed of via wastewater or household waste. Place the packaging and medicines you no longer need in the SIGRE collection point at the pharmacy. If in doubt, ask your pharmacist how to dispose of the packaging and medicines you no longer need. This will help protect the environment.
6. Package contents and additional information
Composition of Nervinex
The active ingredient is brivudine.
Each Nervinex tablet contains 125 mg of brivudine.
The otheringredients are:
Microcrystalline cellulose, lactose monohydrate, povidone K 24-27, magnesium stearate.
Appearance of the product and package contents
Nervinex 125 mg tablets are round, flat, white or almost white, and have beveled edges.
They are presented in a blister pack inside a carton.
Nervinex is available in cartons containing 1 to 7 tablets and in packs containing 5 cartons, each containing 7 tablets.
Not all pack sizes are marketed.
Marketing authorization holder
Laboratori Guidotti, S.p.A. Via Livornese 897, Loc. “La Vettola” (San Piero a Grado, PISA) Italy.
Local representative
GUIDOTTI FARMA, S.L.
Alfons XII, 587 – 08918 Badalona (Barcelona) Spain
Manufacturer
BERLIN-CHEMIE AG, Glienicker Weg 125, D-12489 Berlin, Germany
This medication has been authorized in the Member States of the European Economic Area under the following names:
Germany | Premovir |
Austria | Mevir |
Belgium | Zerpex |
Greece | Brivir |
Italy | Brivirac |
Luxembourg | Zerpex |
Portugal | Bridic |
Spain | Nervinex |
Date of the last revision of this prospectus:February 2021
Detailed and updated information on this medication is available on the website of the Spanish Agency for Medicines and Health Products (AEMPS) http://www.aemps.gob.es/
- Country of registration
- Average pharmacy price66.58 EUR
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to NERVINEX 125 mg TABLETSDosage form: TABLET, 125 mgActive substance: brivudineManufacturer: Aristo Pharma GmbhPrescription requiredDosage form: INJECTABLE PERFUSION, 25 mg/mlActive substance: aciclovirManufacturer: Accord Healthcare S.L.U.Prescription requiredDosage form: INJECTABLE PERFUSION, 250 mg acyclovirActive substance: aciclovirManufacturer: Accord Healthcare S.L.U.Prescription required
Online doctors for NERVINEX 125 mg TABLETS
Discuss questions about NERVINEX 125 mg TABLETS, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions
















