KETISAL 0.25 mg/ml EYE DROPS SOLUTION


How to use KETISAL 0.25 mg/ml EYE DROPS SOLUTION
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the User
Ketisal 0.25 mg/ml Eye Drops Solution
Ketotifen
Read this package leaflet carefully before you start using this medicine because it contains important information for you.
- Keep this package leaflet, you may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their symptoms are the same as yours.
- If you experience any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this package leaflet. See section 4.
Contents of the Package Leaflet
- What is Ketisal 0.25 mg/ml Eye Drops Solution and what is it used for
- What you need to know before you start using Ketisal 0.25 mg/ml Eye Drops Solution
- How to use Ketisal 0.25 mg/ml Eye Drops Solution
- Possible side effects
- Storage of Ketisal 0.25 mg/ml Eye Drops Solution
- Package Contents and Additional Information
1. What is Ketisal and what is it used for
This medicine contains the active substance ketotifen, which is an antiallergic molecule.
This medicine is used to treat the ocular symptoms of hay fever.
2. What you need to know before you start using Ketisal
Do not use Ketisal
- If you are allergic (hypersensitive) to ketotifen or any of the other ingredients of this medicine (listed in section 6).
Warnings and Precautions
Talk to your doctor or pharmacist before you start using this medicine.
Children
Ketisal is not recommended for use in children under 3 years of age.
Other Medicines and Ketisal
If you need to apply any other eye medicine with this medicine, wait at least 5 minutes between applying each product.
Tell your doctor or pharmacist if you are using or have recently used or might use any other medicines, including those obtained without a prescription. This is especially important with medicines used to treat:
- depression, anxiety, and sleep disorders
- allergies (e.g., antihistamines)
Ketisal with Food, Drink, and Alcohol
Using this medicine may increase the effects of alcohol.
Pregnancy and Breastfeeding
If you are pregnant or breastfeeding, think you may be pregnant, or are planning to have a baby, ask your doctor or pharmacist for advice before using this medicine.
This medicine can be used during breastfeeding.
Driving and Using Machines
This medicine may cause blurred vision or drowsiness. If this happens, wait until these effects have gone before driving or using machines.
3. How to Use Ketisal
Follow exactly the administration instructions of this medicine given by your doctor or pharmacist. If in doubt, consult your doctor or pharmacist again.
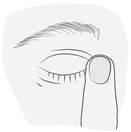
The recommended dose in adults, elderly patients, and children (from 3 years of age), is one drop in the affected eye(s) twice a day (morning and evening).
Instructions for Use
1a
|
|
|
|
|
|
5
|
|
1-5 |
|
If you have any further questions on the use of this medicine, ask your doctor or pharmacist.
If you use more Ketisal than you should
There is no danger if you have used more than one drop in the eye or if you have accidentally ingested this medicine. If in doubt, consult your doctor or pharmacist.
In case of overdose or accidental ingestion, consult your doctor or pharmacist or call the Toxicological Information Service, phone: 91 562 04 20, indicating the medicine and the amount ingested.
If you forget to use Ketisal
If you forget to use this medicine, you should apply the treatment as soon as you remember, and at the recommended dose (one drop per eye, twice a day). Do not use a double dose to make up for forgotten doses.
4. Possible Side Effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
The following side effects have been reported.
Common (may affect up to 1 in 10 people)
- eye irritation or pain in the eye
- inflammation in the eye
Uncommon (may affect up to 1 in 100 people)
- blurred vision when applying the drops in the eye
- dryness in the eye
- alteration in the eyelid
- conjunctivitis
- increased sensitivity of the eyes to light
- visible bleeding in the white part of the eye
- headache
- drowsiness
- rash (which can also cause itching)
- eczema (itching, redness, rash with itching)
- dry mouth
- allergic reaction (including swelling of the face and eyelids) and increased severity of an existing allergic condition such as asthma and eczema.
If you experience serious side effects, talk to your doctor or pharmacist, even if they are not listed in this package leaflet.
Reporting of Side Effects
If you experience any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this package leaflet. You can also report side effects directly through the Spanish Medicines Monitoring System, https://www.notificaram.es. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of Ketisal
Keep this medicine out of the sight and reach of children.
Do not store above 25°C.
Once the bottle is opened, it can be stored for 3 months.
Do not use this medicine after the expiry date which is stated on the carton and the bottle after EXP. The expiry date is the last day of the month stated.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. These measures will help protect the environment.
6. Package Contents and Additional Information
Composition of Ketisal
- The active substance is ketotifen (as hydrogen fumarate).
Each ml of solution contains 0.345 mg of hydrogen fumarate of ketotifen, equivalent to 0.25 mg of ketotifen.
- The other ingredients (excipients) are sodium hyaluronate, glycerol (E 422), sodium hydroxide (E 524), and purified water.
Appearance and Package Contents
This medicine is a clear, colorless or slightly yellowish solution. The solution is filled in a 10 ml white plastic bottle with a dropper. Each plastic bottle contains 10 ml of solution.
Marketing Authorization Holder
HORUS PHARMA
22 Allé Camille Muffat
INEDI 5
06200 Nice
France
Manufacturer
PHARMASTER
Zone Industrielle de Krafft Erstein
FRANCE
This medicine is authorized in the Member States of the European Economic Area under the following names:
Germany: Ketazed 0.25 mg/ml, Augentropfen, Lösung
Belgium: Ketazed 0.25 mg/ml collyre en solution / Ketazed 0.25 mg/ml oogdruppels, oplossing / Ketazed 0.25 mg/ml Augentropfen, Lösung
Denmark: Ketazed, øjendråber, opløsning
Spain: Ketisal 0.25 mg/ml colirio en solución
Finland: Ketazed 0.25 mg/ml silmätipat, liuos
France: Ketazed 0.25 mg/mL, collyre en solution
Netherlands: Ketazed 0.25 mg/ml oogdruppels, oplossing
Italy: KETAZED 0.25 mg/ml, collirio, soluzione
Luxembourg: Ketazed 0.25 mg/ml collyre en solution
Norway: Ketazed 0.25 mg/ml øyedråper, oppløsning
Romania: Ketazed 0.25 mg/ml picaturi oftalmice, solu?ie
Sweden: Ketazed 0.25 mg/ml ögondroppar, lösning
You can request more information about this medicine by contacting the local representative of the marketing authorization holder:
Horus Pharma Ibérica, S.L.U.
Gran Vía Carlos III, 98, 6º
08028 Barcelona - Spain
Date of the Last Revision of this Package Leaflet: June 2025
Detailed information on this medicine is available on the website of the Spanish Agency for Medicines and Health Products (AEMPS) http://www.aemps.gob.es/
- Country of registration
- Average pharmacy price7.02 EUR
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to KETISAL 0.25 mg/ml EYE DROPS SOLUTIONDosage form: EYEDROP, 0.25 mg/mlActive substance: ketotifenManufacturer: Laboratoires TheaPrescription requiredDosage form: EYEDROP, 0.25mg/mlActive substance: ketotifenManufacturer: Pharma Stulln GmbhPrescription requiredDosage form: EYE DROP, 0.25 mg/mlActive substance: ketotifenManufacturer: Nutra Essential Otc S.L.Prescription required
Online doctors for KETISAL 0.25 mg/ml EYE DROPS SOLUTION
Discuss questions about KETISAL 0.25 mg/ml EYE DROPS SOLUTION, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions




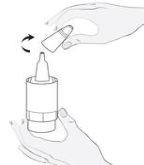 1b
1b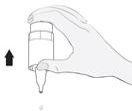 2
2 3
3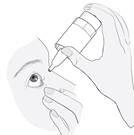 4
4