
KENTERA 3.9 mg/24 hours, transdermal patch


How to use KENTERA 3.9 mg/24 hours, transdermal patch
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the User
Kentera 3.9 mg / 24 hours, transdermal patch
oxybutynin
Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the pack
- What is Kentera and what is it used for
- What you need to know before you use Kentera
- How to use Kentera
- Possible side effects
- Storing Kentera
- Contents of the pack and further information
1. What is Kentera and what is it used for
Kentera is used in adults to control the symptoms of urgency incontinence and/or increased frequency and urgency of urination.
Kentera works by allowing the bladder to expand and hold more urine.
2. What you need to know before you use Kentera
Do not useKentera
- if you are allergic to oxybutynin or any of the other ingredients of this medicine (listed in section 6).
- if you have a rare disease called myasthenia gravis that makes the muscles in your body weak and tire easily.
- if you have a condition where your bladder does not empty completely when you urinate. Using oxybutynin may make this problem worse. You should discuss this with your doctor before using Kentera.
- if you have digestive problems due to a reduction in stomach emptying after meals, you should consult your doctor before using Kentera.
- if you have glaucoma or a family history of glaucoma, inform your doctor.
Warnings and precautions
Consult your doctor or pharmacist before starting to use Kentera if you have any of the following:
- Liver problems
- Kidney problems
- Difficulty urinating
- Intestinal obstruction
- Blood in your stools
- Generalized muscle weakness
- Pain when swallowing
As treatment with oxybutynin can reduce sweating, the risk of fever and heat stroke is higher when the ambient temperature is higher.
Children and adolescents
The use of Kentera is not recommended in children or adolescents.
Other medicines and Kentera
Tell your doctor or pharmacist if you are using, have recently used, or might use any other medicines.
Using the Kentera patch at the same time as taking other medicines that have similar side effects, such as dry mouth, constipation, and drowsiness, may increase the frequency or severity of these side effects.
Oxybutynin may slow down the functioning of the digestive tract and thus affect the absorption of other oral medicines. On the other hand, the use of this medicine with other medicines may increase the effect of oxybutynin, especially:
- Ketoconazole, itraconazole, or fluconazole (used to treat fungal infections).
- Erythromycin, a macrolide antibiotic (used to treat bacterial infections).
- Biperiden, levodopa, or amantadine (used to treat Parkinson's disease).
- Antihistamines (used in the treatment of allergies such as hay fever).
- Phenothiazines or clozapine (used to treat mental illnesses).
- Tricyclic antidepressants (used to treat depression).
- Dipyridamole (used to treat blood coagulation problems).
- Atropine and other anticholinergics (used to treat stomach disorders such as irritable bowel syndrome).
Using Kentera with alcohol
Oxybutynin may cause drowsiness and blurred vision. Consuming alcohol may increase drowsiness.
Pregnancy and breastfeeding
If you are pregnant or breastfeeding, think you may be pregnant, or plan to become pregnant, consult your doctor or pharmacist before using this medicine.
Kentera should not be used during pregnancy unless clearly necessary.
When oxybutynin is used during breastfeeding, a small amount is excreted in the mother's milk. Therefore, the use of oxybutynin is not recommended during breastfeeding.
Driving and using machines
Since Kentera can cause drowsiness, somnolence, or blurred vision, patients should be advised to be cautious when driving and using machines.
3. How to use Kentera
Follow the instructions for administration of this medicine exactly as indicated by your doctor. If in doubt, consult your doctor or pharmacist again.
Apply a new Kentera patch twice a week (every 3 or 4 days) in the indicated form. Always change the patch on the same two days, for example on Sundays and Wednesdays, or on Mondays and Thursdays.
Inside the Kentera package, you will find a calendar to help you remember when it's time for your dose. Mark the days you have decided to apply the medicine and do not forget to change the patch always on the same two days of the week you have chosen. Make sure you only have one patch on your body at a time and keep it applied permanently until it's time to change it for a new one.
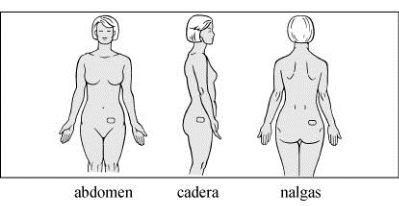
Where to apply
Apply the patch to a clean, dry, and smooth area of the skin on the abdomen, hip, or buttocks. It is not recommended to apply the patch to the waist due to the risk of rubbing against tight clothing. Do not expose to the sun. Place the patch under clothing. Alternate application sites with each new application. Do not apply another patch to the same site for at least one week.
How to apply
Each patch is individually wrapped in a protective envelope. Read the following instructions before applying Kentera for the first time.
To applyKentera
Step 1: Choose a suitable place to apply the patch.
- Freshly washed skin, but dry and cool (wait a few minutes after a warm bath or shower).
- Where you have not applied talc, lotions, or body oils.
- Where you do not have cuts, eruptions, or other forms of skin irritation.
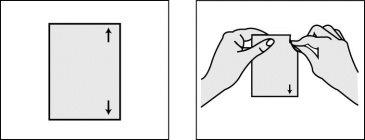
Step 2: Open the envelope containing the patch.
- Break the bag at the arrows located on the right side of the envelope, as indicated in the drawing.
- Do not use scissors to cut the bag: you could damage the patch.
- Remove the patch from the bag.
- Do not cut or divide the patch. Do not use damaged patches.
- Apply it immediately to the skin; do not keep the patch outside the airtight envelope.
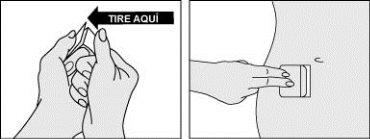
Step 3: Apply half of the patch to the skin.
- Carefully fold the patch and remove one half of the protector covering the adhesive surface of the patch.
- Without touching the adhesive surface, apply the adhesive part to the skin of the selected area on the abdomen, hip, or buttocks and press.
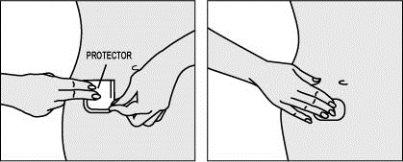
Step 4: Apply the other half of the patch to the skin.
- Fold the patch over itself, pressing on the covering.
- Gently pull the covering to lift the edge.
- Hold the edge by one of its corners and remove the second half of the protector. Try not to touch the adhesive part of the patch.
- Press with your fingers over the entire patch for at least 10 seconds to secure it well. Make sure the entire patch is stuck to the skin, including the edges.
- Discard the protective coverings.
Bathing, showering, swimming, and exercise
You should wear the patch continuously until you apply a new one. Bathing, showering, swimming, and exercise will not affect the patch as long as you do not rub it when washing. Avoid staying in the bathtub for a long time, as the patch may come off.
If the patch comes off
If the patch starts to separate from the skin, apply gentle pressure with your fingers. The patch is designed to re-adhere. In rare cases, the patch may come off completely. In this case, try to put the patch back in the same place. If the entire patch adheres firmly, leave it on. If not, remove it and apply a new patch to a different area. Regardless of the day it happens, continue with the same twice-weekly schedule you have marked on the patch box.
If you forget to change the patch after 3-4 days
As soon as you remember, remove the old patch and apply a new one to a different area of your abdomen, hip, or buttocks. Regardless of the day it happens, continue with the same twice-weekly schedule for your next patch, even if you have to change it before 3 or 4 days have passed.
How to remove it
To change it, slowly remove the used patch. Fold it in half (with the adhesive surface inward) and dispose of it in a way that is out of the reach of children and pets. The application site may be slightly reddened, but the redness should disappear a few hours after removing the patch. Consult your doctor if the irritation persists.
Normally, the adhesive residue from the removed patch can be removed by gently washing the skin with warm water and a mild soap. It can also be cleaned with a little baby oil. To remove any remaining adhesive marks, it may be necessary to use a special wipe for removing adhesive residue (available at pharmacies). Do not use alcohol or other strong solvents that may irritate the skin.
After use, the patch still contains significant amounts of active ingredients that can be harmful to the aquatic environment. Therefore, after removal, the used patch should be folded in half, with the adhesive surface inward so that the release membrane is not exposed, placed in its original envelope, and then disposed of safely and out of the reach of children. All patches, both used and unused, should be disposed of in accordance with local regulations or returned to the pharmacy. Used patches should not be thrown into the toilet or disposed of in liquid waste disposal systems.
If you use more Kentera than you should
Do not apply more than one patch to your body at a time.
If you forget to use Kentera
Apply a Kentera patch as soon as you realize you are not wearing one, or if you have missed one of the days marked on the calendar.
If you stop using Kentera
Your urgency incontinence may return, and you may experience an increase in urination frequency if you decide to stop using the patch. Continue using Kentera while your doctor does not indicate otherwise.
If you have any other questions about the use of this medicine, ask your doctor or pharmacist.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Very commonside effects(may affect more than 1 in 10 people)
- Itching around the application site
Commonside effects(may affect up to 1 in 10 people)
- Redness or rash at the application site
- Dry mouth
- Constipation
- Diarrhea
- Stomach discomfort
- Stomach pain
- Headache or drowsiness
- Urinary tract infections
- Blurred vision
- Dizziness
Uncommonside effects(may affect up to 1 in 100 people)
- Fungal or upper respiratory tract infections
- Anxiety
- Confusion
- Nervousness
- Agitation
- Difficulty sleeping
- Palpitations
- Hot flashes
- Back pain
- Urinary retention
- Difficulty urinating
- Cold
- Accidental injury
Rareside effects(may affect up to 1 in 1,000 people)
- Panic reaction
- Mental confusion
- Hallucinations
- Disorientation
- Memory impairment
- Memory loss
- Abnormal fatigue
- Poor concentration
Reporting of side effects
If you experience any side effects, talk to your doctor or pharmacist, even if it is possible side effects not listed in this leaflet. You can also report side effects directly through the national reporting system included in Appendix V. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storing Kentera
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date which is stated on the envelope and the carton. The expiry date is the last day of the month stated.
Do not refrigerate or freeze.
Used patches should be folded in half, with the adhesive surface inward so that the release membrane is not exposed, placed in their original envelope, and then disposed of safely out of the reach of children. All patches, both used and unused, should be disposed of in accordance with local regulations or returned to the pharmacy. Used patches should not be thrown into the toilet or disposed of in liquid waste disposal systems.
6. Package Contents and Additional Information
Kentera Composition
- The active ingredient is oxybutynin.
Each transdermal patch releases 3.9 mg of oxybutynin every 24 hours. Each 39 cm2 patch contains 36 mg of oxybutynin.
- The other components are: triacetin and acrylic adhesive solution. The oxybutynin, triacetin, and acrylic adhesive have a transparent PET/EVA reinforcing film and are coated with a silicone-coated polyester release liner.
Product Appearance and Package Contents
Kentera is a transdermal patch that comes in boxes of 2, 8, or 24 patches.
Each patch is covered with a protective reinforcing film on the side of the patch that is coated with the active ingredients. The reinforcing film should be removed before applying the patch.
Marketing Authorization Holder
Teva B.V.
Swensweg 5
2031 GA Haarlem
Netherlands
Manufacturer
Merckle GmbH
Ludwig-Merckle-Straße 3
89143 Blaubeuren
Germany
Teva Pharmaceuticals Europe B.V.
Swensweg 5
2031 GA Haarlem
Netherlands
You can request more information about this medication by contacting the local representative of the marketing authorization holder.
Belgium/Belgique/Belgien Teva Pharma Belgium N.V./S.A./AG Tel: +32 38207373 | Lithuania UAB Teva Baltics Tel: +370 52660203 |
Bulgaria Pharmasport Ltd. Tel: +359 24899585 | Luxembourg/Luxemburg Teva Pharma Belgium N.V./S.A./AG Belgium/Belgien Tel: +32 38207373 |
Czech Republic Teva Pharmaceuticals CR, s.r.o. Tel: +420 251007111 | Hungary Teva Gyógyszergyár Zrt. Tel: +36 12886400 |
Denmark Teva Denmark A/S Tlf: +45 44985511 | Malta Teva Pharmaceuticals Ireland Ireland Tel: +44 2075407117 |
Germany ratiopharm GmbH Tel: +49 73140202 | Netherlands Teva Nederland B.V. Tel: +31 8000228400 |
Estonia UAB Teva Baltics Eesti filiaal Tel: +372 6610801 | Norway Teva Norway AS Tlf: +47 66775590 |
Greece Specifar A.B.E.E. Tel: +30 2118805000 | Austria ratiopharm Arzneimittel Vertriebs-GmbH Tel: +43 1970070 |
Spain Laboratorios Gebro Pharma, S.A. Tel: +34 932058686 | Poland Teva Pharmaceuticals Polska Sp. z o.o. Tel: +48 223459300 |
France Teva Santé Tél: +33 155917800 | Portugal Teva Pharma - Produtos Farmacêuticos, Lda. Tel: +351 214767550 |
Croatia Pliva Hrvatska d.o.o. Tel: +385 13720000 | Romania Teva Pharmaceuticals S.R.L. Tel: +40 212306524 |
Ireland Teva Pharmaceuticals Ireland Tel: +44 2075407117 | Slovenia Pliva Ljubljana d.o.o. Tel: +386 15890390 |
Iceland Teva Pharma Iceland ehf. Sími: +354 5503300 | Slovakia TEVA Pharmaceuticals Slovakia s.r.o. Tel: +421 257267911 |
Italy Teva Italia S.r.l. Tel: +39 028917981 | Finland Teva Finland Oy Puh/Tel: +358 201805900 |
Cyprus Specifar A.B.E.E. Greece Tel: +30 2118805000 | Sweden Teva Sweden AB Tel: +46 42121100 |
Latvia UAB Teva Baltics filiale Latvija Tel: +371 67323666 | United Kingdom (Northern Ireland) Accord Healthcare Ireland Ltd. Ireland Tel: +353 214619040 |
Date of Last Revision of this Leaflet:
Detailed information on this medicinal product is available on the European Medicines Agency website: http://www.ema.europa.eu
- Country of registration
- Average pharmacy price40.59 EUR
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to KENTERA 3.9 mg/24 hours, transdermal patchDosage form: TABLET, 5 mgActive substance: oxybutyninManufacturer: Cheplapharm Arzneimittel GmbhPrescription requiredDosage form: VESICAL IRRIGATION SOLUTION, 1 mg/mlActive substance: oxybutyninManufacturer: Farco-Pharma GmbhPrescription requiredDosage form: MODIFIED-RELEASE TABLET, 50 mgActive substance: mirabegronManufacturer: Laboratorios Alter S.A.Prescription required
Online doctors for KENTERA 3.9 mg/24 hours, transdermal patch
Discuss questions about KENTERA 3.9 mg/24 hours, transdermal patch, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions






